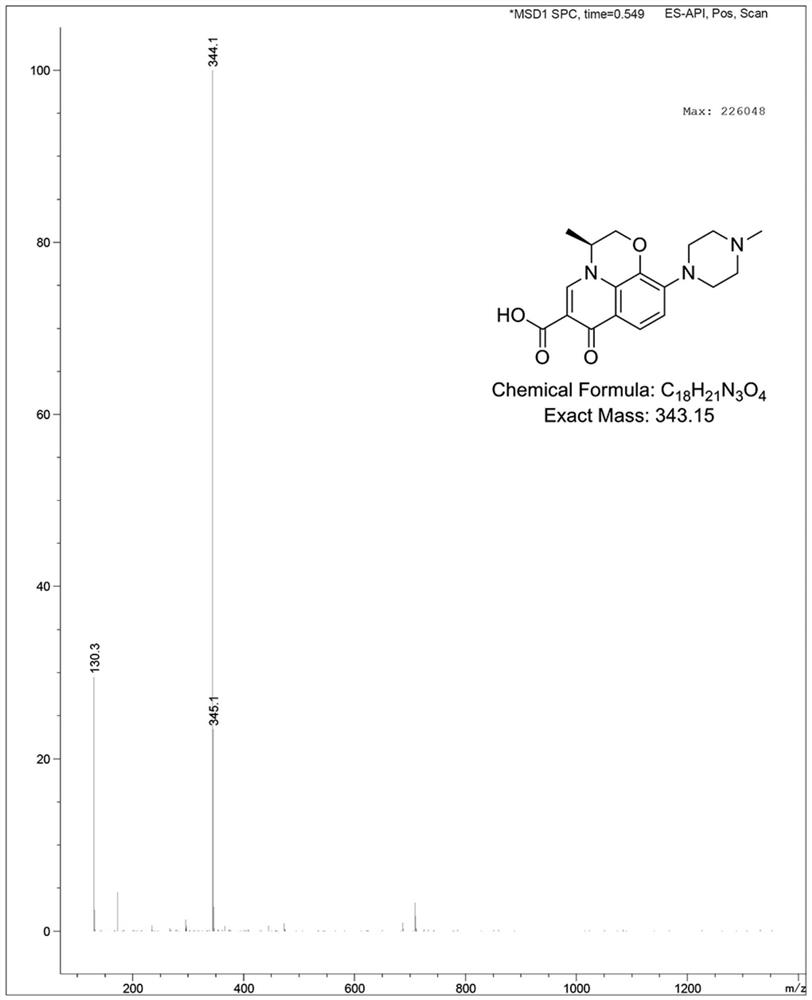
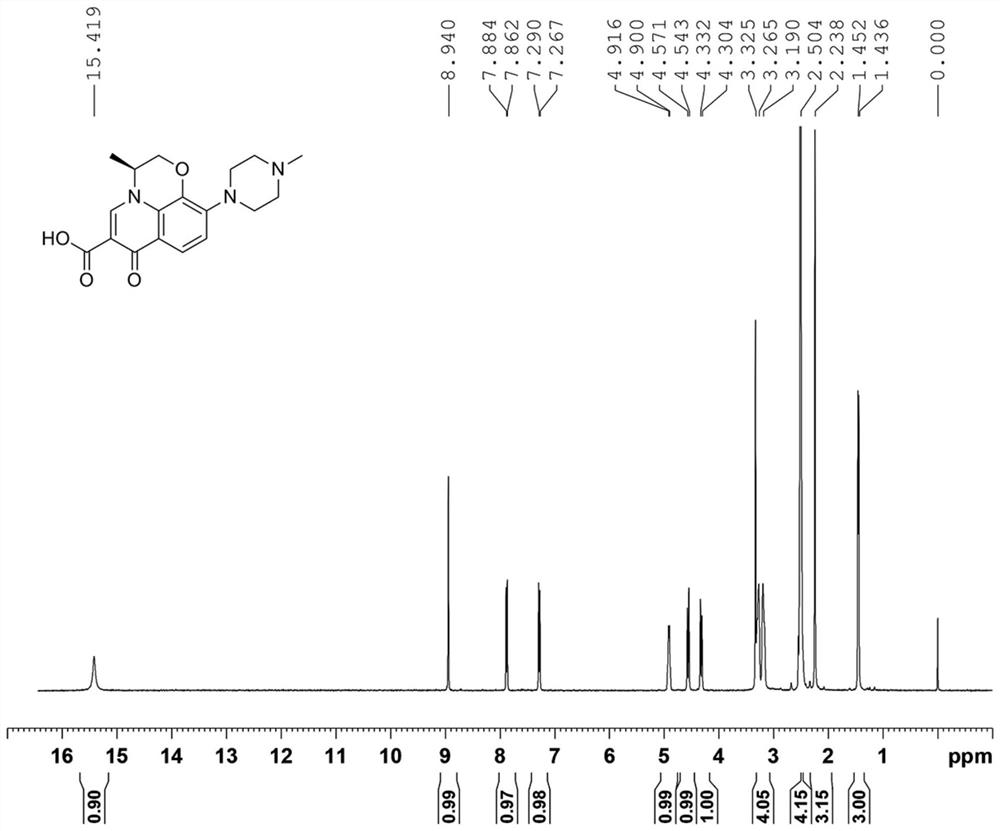
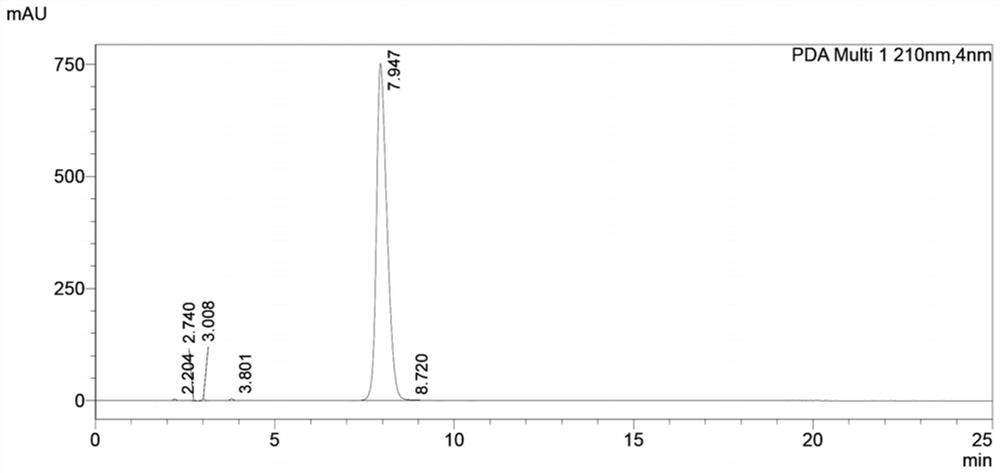
Preparation method of levofloxacin defluorination impurity
A technology of levofloxacin and defluorination, which is applied in the field of preparation of pharmaceutical impurity standard products, can solve the problems of long steps and high requirements for operators, and achieve the effects of short preparation cycle, high purity and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of levofloxacin defluorination impurity
[0033] Preparation of compound (Ⅲ):
[0034] Add sodium methylthiolate (12.6g, 180 mmol, 6.0 eq) to a solution of levofloxacin (10.83g, 30 mmol, 1.0 eq) dissolved in N,N-dimethylformamide (86 mL), and then at 70°C Stir for 48 h. After the TLC plate showed that the reaction of the raw materials was complete, 200 mL of water was added to the above reaction solution. Finally, the mixture was stirred down to room temperature, and a large amount of off-white solid was precipitated, which was filtered by suction and rinsed with an appropriate amount of water and a small amount of ethanol. The filter cake was collected and dried to obtain 11.11 g of compound (Ⅲ), an off-white solid, with a yield of 95.1%.
[0035] Preparation of levofloxacin defluorinated impurities:
[0036] Add Raney nickel (29 mg, 0.5 mmol, 0.05 eq) to a solution of compound (Ⅲ) (3.89 g, 10 mmol, 1.0 eq) in ethanol (78 mL), and then ...
Embodiment 2
[0046] Embodiment 2: the preparation of levofloxacin defluorination impurity
[0047] Preparation of compound (Ⅲ):
[0048] Sodium methylthiolate (14.7g, 210 mmol, 7.0 eq) was added to levofloxacin (10.83g, 30 mmol, 1.0 eq) dissolved in dimethyl sulfoxide (54 mL), and stirred at 100°C for 48 h. After the TLC plate showed that the reaction of the raw materials was complete, 200 mL of water was added to the above reaction solution. Finally, the mixture was stirred down to room temperature, and a large amount of off-white solid was precipitated. The filter cake was collected by suction filtration and rinsed with an appropriate amount of water and a small amount of ethanol. After drying, 10.52 g of compound (Ⅲ) was obtained as an off-white solid with a yield of 90.0%.
[0049] Preparation of levofloxacin defluorinated impurities:
[0050] Add Raney nickel (59 mg, 1 mmol, 0.1 eq) to a solution of compound (Ⅲ) (3.89 g, 10 mmol, 1.0 eq) in methanol (39 mL), and then wash with N 2 Re...
Embodiment 3
[0051] Embodiment 3: the preparation of levofloxacin defluorination impurity
[0052] Preparation of compound (Ⅲ):
[0053] Add sodium methylthiolate (6.3g, 90 mmol, 3.0 eq) to a solution of levofloxacin (10.83g, 30 mmol, 1.0 eq) in N-methylpyrrolidone (108 mL), then stir at 50°C for 48 h . After the TLC plate showed that the reaction of the raw materials was complete, 150 mL of water was added to the above reaction solution. Finally, the mixture was stirred down to room temperature, and a large amount of off-white solid was precipitated. The filter cake was collected by suction filtration and rinsed with an appropriate amount of water and a small amount of ethanol. After drying, 8.95 g of compound (Ⅲ) was obtained as an off-white solid with a yield of 76.6%.
[0054] Preparation of levofloxacin defluorinated impurities:
[0055] Add Raney nickel (18 mg, 0.3 mmol, 0.03 eq) to a solution of compound (Ⅲ) (3.89 g, 10 mmol, 1.0 eq) in isopropanol (78 mL), and then wash with N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com