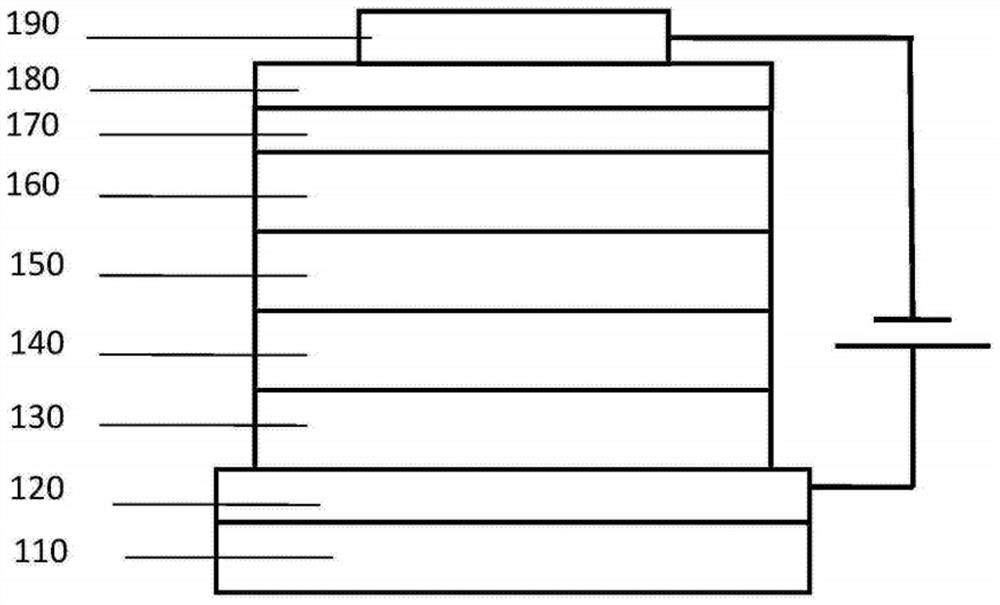
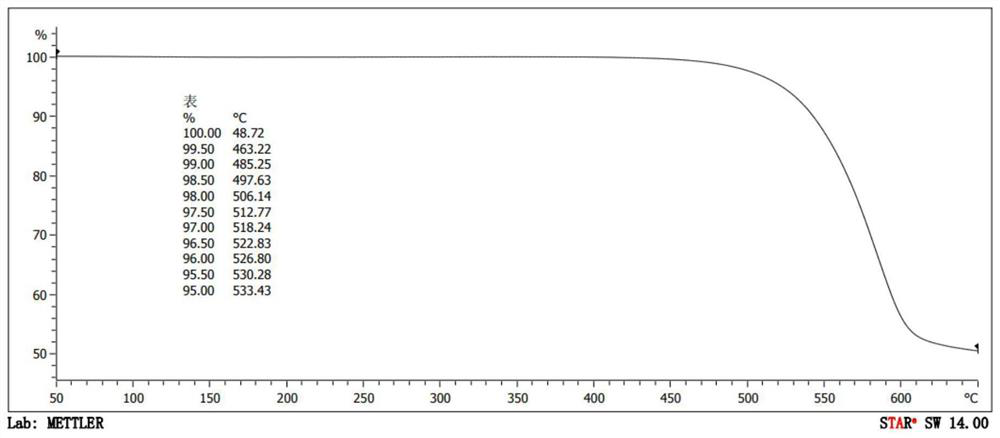
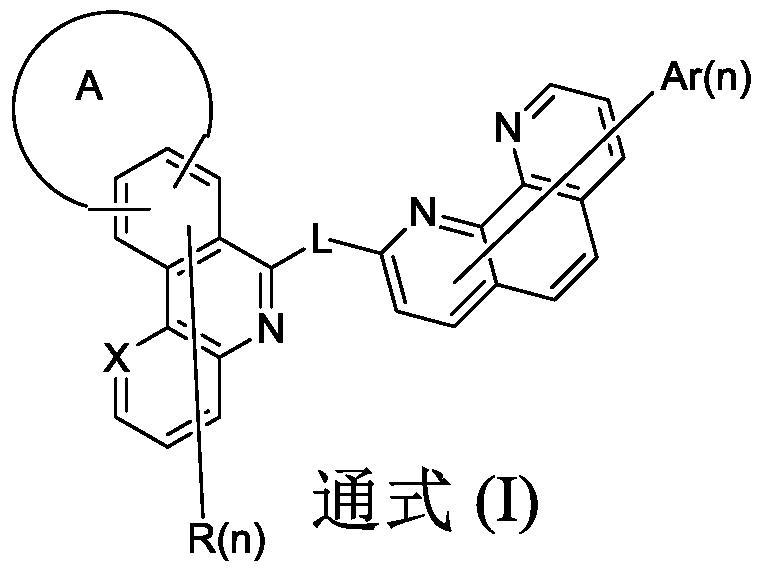
Organic compound and organic electroluminescent device using same
An organic compound and compound technology, applied in the field of organic electroluminescent devices, can solve the problems of further improvement in stability, and achieve the effects of not being easy to crystallize and decompose, poor spatial symmetry, and increasing the glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of compound C-1
[0057] (1) Preparation of Intermediate-1
[0058]
[0059] Under a nitrogen atmosphere, 2-chloro-3-aminopyridine (11 grams), dibenzofuran-4-boronic acid (21 grams), tetrahydrofuran (120 milliliters) were charged into a reaction vessel, and potassium carbonate (15.7 g) was dissolved in water (56 mL) to form an aqueous solution, palladium acetate (0.5 g) was added, and triphenylphosphine (2.1 g) was stirred under reflux for one night. After natural cooling, ethyl acetate was added into the system for extraction operation, and the organic layer was concentrated to obtain a crude product. Petroleum ether was added to the crude product, and the precipitated solid was filtered and taken out, thus obtaining 24.6 g of 2-(dibenzofuran-4-yl)-3-aminopyridine (intermediate-1) (yield 94%) ).
[0060] (2) Preparation of Intermediate 2
[0061]
[0062] Charge intermediate-1 (13 grams), triethylamine (5 grams), dichloromethane ...
Embodiment 2
[0069] Embodiment 2: the synthesis of compound C-2
[0070]
[0071] Similar to the synthetic route of compound C-1, the dibenzofuran-4-boronic acid in the synthesis of intermediate-1 is replaced with dibenzothiophene-4-boronic acid to obtain intermediate-4, intermediate-5, intermediate -6, and compound C-2, the yield of intermediate-6 to compound C-2 is 73%; LC-MS: M / Z 617.2 (M+H) + .
Embodiment 3
[0072] Embodiment 3: the synthesis of compound C-3
[0073]
[0074] Similar to the synthetic route of compound C-1, the dibenzofuran-4-boronic acid in the synthesis of intermediate-1 is replaced with 9,9-dimethylfluorene-1-boronic acid, and intermediate-7, intermediate- 8, intermediate-9, and compound C-3, the yield of intermediate-9 to compound C-3 is 83%; LC-MS: M / Z 627.3 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com