Condensed ring organic compound and organic electroluminescent device using compound
An organic compound and luminescent technology, which is applied in the direction of silicon organic compounds, organic chemistry, and electric solid-state devices, can solve the problem of unsatisfactory hole-blocking materials, which are not conducive to high-efficiency green phosphorescent devices, and the stability needs to be further improved. and other problems, to achieve the effect of not easy to crystallize and decompose, the synthesis method is simple, and the lifespan is improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
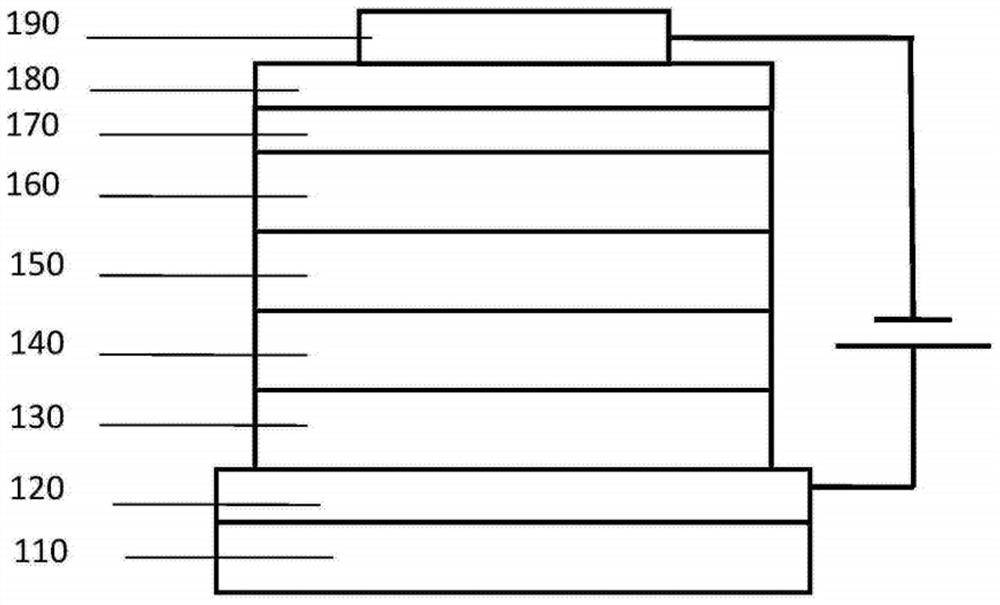
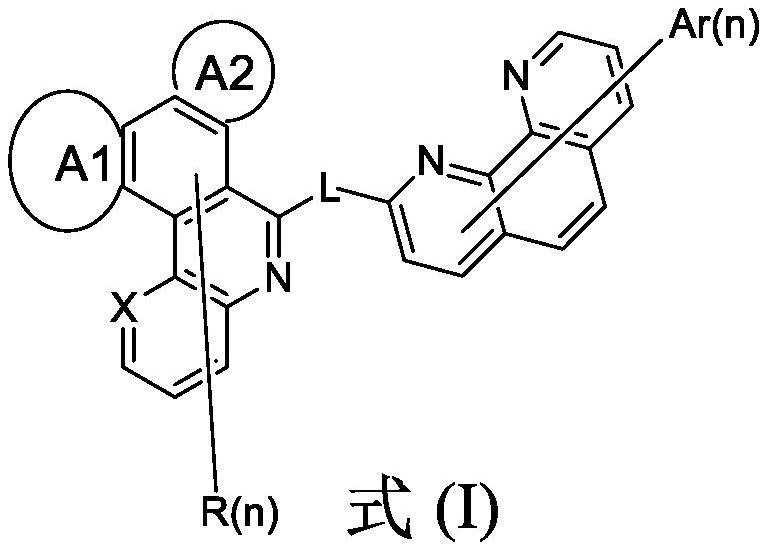
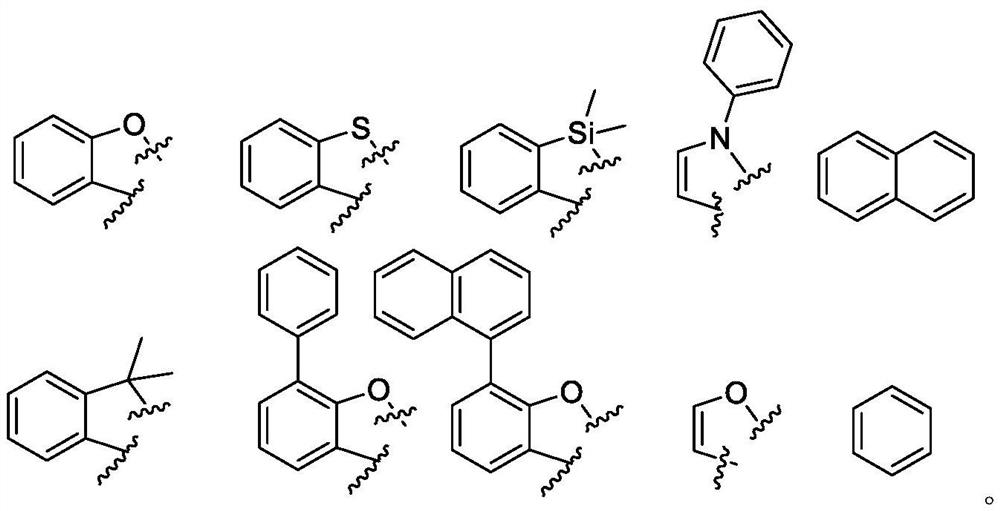
Image
Examples
Embodiment 1
[0052] Embodiment 1: the preparation of compound C-19
[0053] (1) Preparation of intermediate sub-1
[0054]
[0055] 2-Chloro-3-aminopyridine: 11.0g (51.87mmol), phenanthrene-9-boronic acid: 11.52g, toluene: 105mL, ethanol: 26mL were charged in the reaction vessel, and then potassium carbonate: 15.7g was added to dissolve at H 2 O: 56 mL of the aqueous solution was irradiated with ultrasonic waves for 30 minutes while passing nitrogen gas. Add palladium acetate: 0.6 g, and stir overnight under heating and reflux. After natural cooling, ethyl acetate was added to the system for extraction, and the organic layer was concentrated to obtain a crude product. n-heptane was added to this crude product, and the precipitated solid was collected by filtration to obtain 2-(phenanthrene-9-yl)-3-aminopyridine (sub-1)13.5 (yield 96%).
[0056] (2) Preparation of intermediate sub-2
[0057]
[0058] Put intermediate sub-1: 22.5g, triethylamine: 9.6g, dichloromethane: 225ml in th...
Embodiment 2
[0065] Embodiment 2: the preparation of compound C-1
[0066]
[0067] Similar to the synthetic route of compound C-19, the dibenzofuran-4-boronic acid in the synthesis of intermediate-1 is replaced by dibenzothiophene-4-boronic acid with oxafluorenofuran-boronic acid, and after 4 steps of reaction, the obtained Compound C1, the yield of the last step reaction is 66%; LC-MS: M / Z 691.2 (M+H) + .
Embodiment 3
[0068] Embodiment 3: the preparation of compound C37
[0069]
[0070] Similar to the synthetic route of compound C-19, the dibenzofuran-4-boronic acid of the synthetic intermediate Sub-1 was replaced with naphthalene-[2,1-b]-benzofuran-6-boronic acid to synthesize the intermediate Sub -4 is replaced by 2-bromo-9-naphthyl-1,10-phenanthroline, and through 4 steps of reaction, to compound C-37, the yield of the last step is 63%; LC-MS: M / Z 701.2 (M +H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com