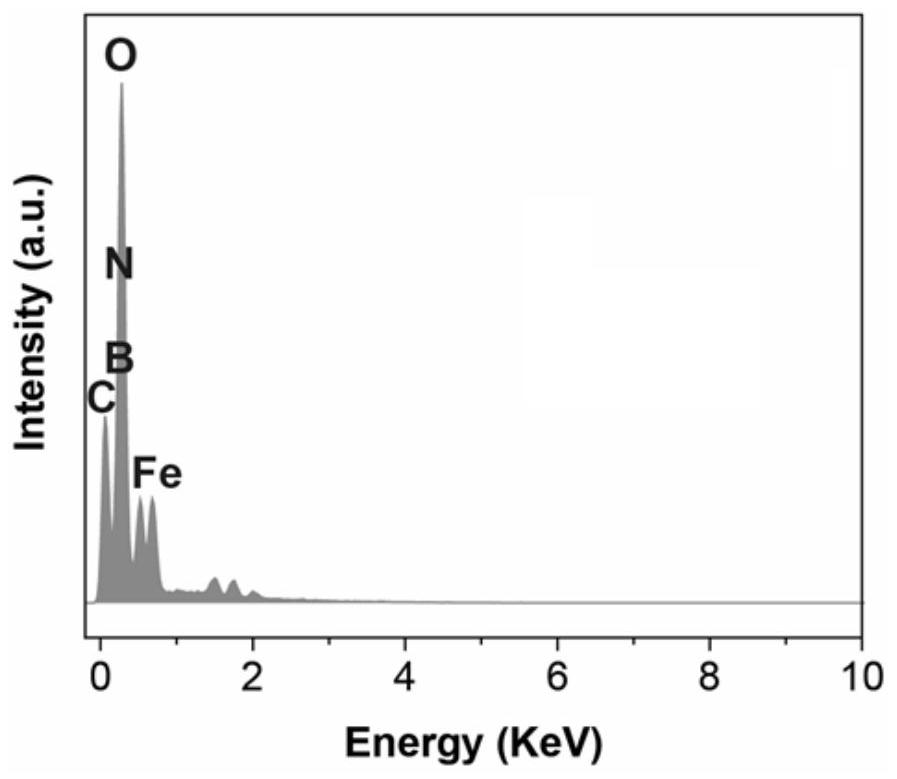
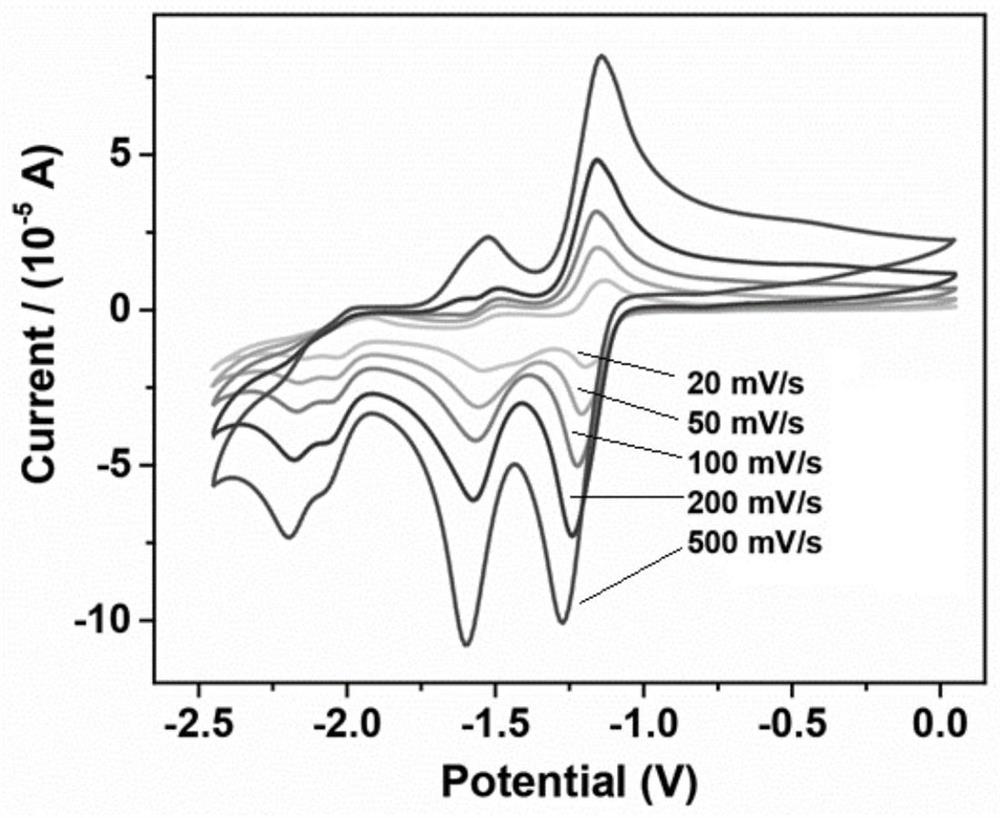
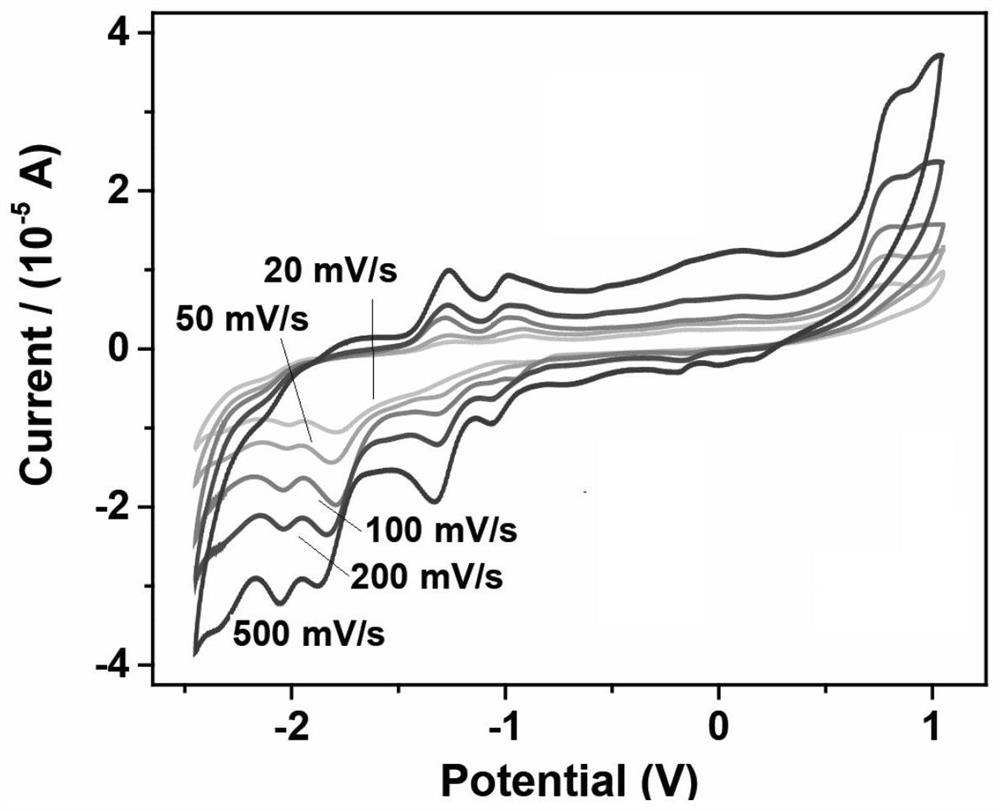
A class of carborane viologen derivatives and their metal supramolecular polymers, synthesis methods and applications
A carborane and viologen technology, used in electrochromic devices and photocatalytic applications, can solve problems such as low electron transfer rate and weak visible light absorption, and achieve improved electron transport performance, efficient intramolecular electron transfer, and improved Effects of Absorptivity and Range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] 1, the synthetic method of a class of carborane viologen derivatives of the present invention, comprises the following steps:
[0061] 1) Synthesis of precursors A and B of carborane viologen derivatives;
[0062]
[0063] Under the protection of an inert gas, every 10mmol (ie 2.82g) reactant 1-iodo-4-bromobenzene and 1-1.2 equivalents of 4-alkynylpyridine, accounting for 10%-15% of the total moles of catalyst bistri Phenylphosphine palladium dichloride is added in the branch bottle that fills 80-100mL acetonitrile / triethylamine (volume ratio is 1 / 1) solvent, and reaction temperature is 70-80 degree, stirs 24-36 hour, through Sonagashira coupling Combined reaction to synthesize 1,2-bis(4-pyridine)acetylene, namely compound 1.
[0064] Then use the reactant decanaborane as an electrophile to attack the alkyne group in compound 1 to synthesize the carborane viologen precursor A, specifically, under the protection of an inert gas, dissolve decanaborane in toluene, and ...
Embodiment 1
[0092] Under the protection of an inert gas, the reactant 1-iodo-4-bromobenzene (10mmol, 2.82g) and 12mmol of 4-alkynylpyridine, the catalyst bistriphenylphosphine palladium dichloride, which accounted for 10% of the total moles, was added 80mL of acetonitrile / triethylamine (volume ratio is 1 / 1) solvent in a branch bottle, the reaction temperature is 75 degrees, stirred for 24 hours, synthesized 1,2-bis(4-pyridine)acetylene by Sonagashira coupling reaction , namely compound 1;
[0093] Then under the protection of an inert gas, dissolve borane in toluene, then add N,N-dimethylaniline and stir, then add 1,2-bis(4-pyridine)acetylene, borane, N,N- The molar ratio of dimethylaniline and 1,2-bis(4-pyridine)acetylene is 1.3:1.5:1, react at 110°C for 48 hours to obtain a reaction solution, separate the product in the reaction solution and dry it. The separation process is that the reaction solution is cooled to room temperature, the insoluble product is removed by filtration, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com