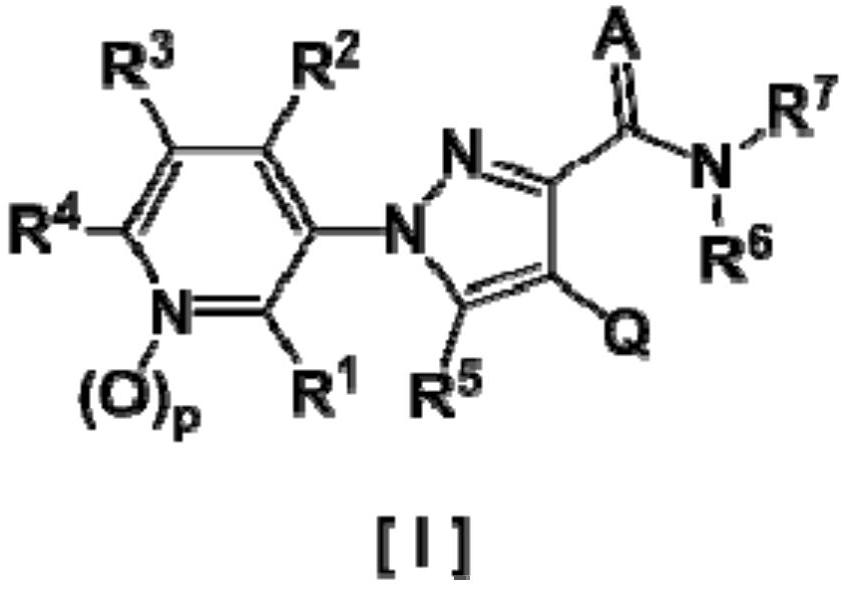
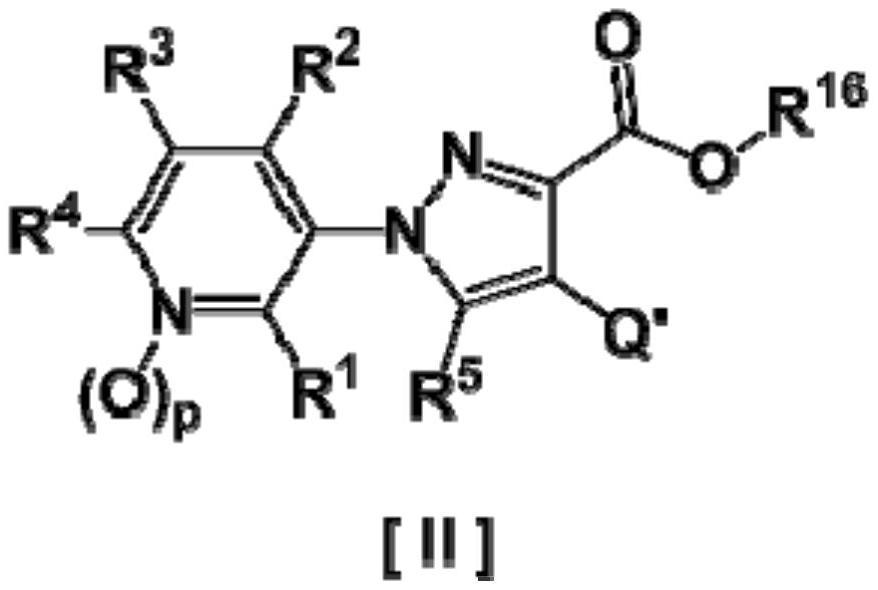
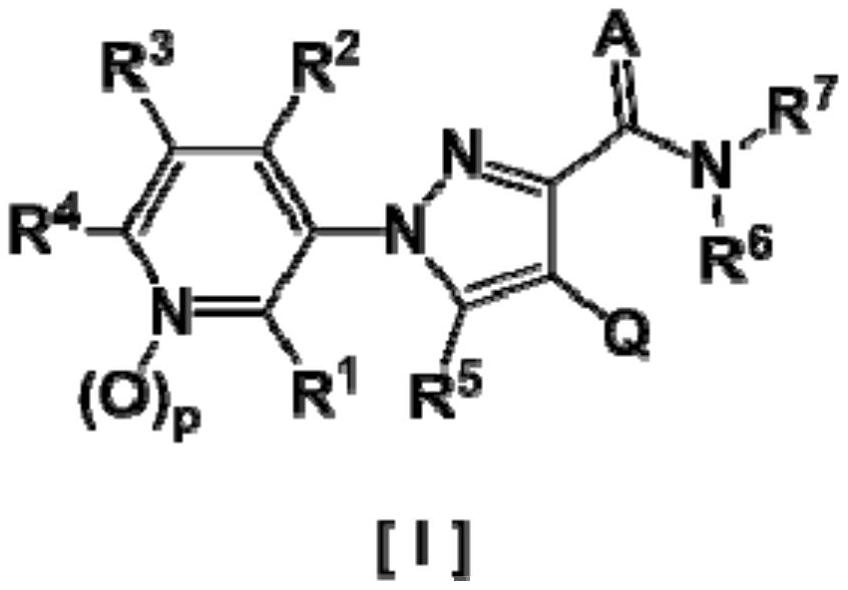
Pyrazole derivative and harmful organism-controlling agent
A technology of pyrazole derivatives and alkyl groups, applied in biocides, animal repellents, plant growth regulators, etc., can solve the problems of unrecorded pest control activity and unrevealed carboxylic acid amides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1059]Production of 1-(pyridin-3-yl)-4-[2-(trifluoromethyl)phenyl]-1H-pyrazole-3-carboxamide (the compound number of the present invention: A-0044)
[1060]1) 4-{[(Nafluorobutyl)sulfonyl]oxy}-1-(pyridin-3-yl)-1H-pyrazole-3-carboxylic acid ethyl ester (the compound number of the present invention: D-0008)
[1061]4-hydroxy-1-(pyridin-3-yl)-1H-pyrazole-3-carboxylic acid ethyl ester (15.0g, 64.3mmol) synthesized by the methods described in Reference Examples 1 and 2 of WO 2016 / 027790A , Triethylamine (13.0 g, 128.5 mmol) and nonafluorobutanesulfonic acid fluoride (27.2 g, 90.0 mmol) were sequentially added to dichloromethane (300 mL) under ice cooling, and stirred at room temperature overnight. The reaction solution was poured into saturated brine, and extracted with dichloromethane. After drying the obtained organic layer with anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure. The residue was washed with diisopropyl ether to obtain 4-{[(nonafluorobutyl)sulfon...
Embodiment 2
[1073]Production of 4-(4-methylpyridin-3-yl)-1-(pyridin-3-yl)-1H-pyrazole-3-carboxamide (the compound number of the present invention: B-100)
[1074]1) 4-(4-Methylpyridin-3-yl)-1-(pyridin-3-yl)-1H-pyrazole-3-carboxylic acid ethyl ester (the compound number of the present invention: D-0108)
[1075]The 4-{[(nonafluorobutyl)sulfonyl]oxy}-1-(pyridin-3-yl)-1H-pyrazole-3-carboxylic acid ethyl ester (3.4g, 6.6mmol), 4-( Methylpyridin-3-yl)boronic acid (1.0g, 7.3mmol), sodium carbonate (1.6g, 15.1mmol) and tetrakis (triphenylphosphine) palladium (0.38g, 0.33mmol) are added to 1,2-dimethyl In a mixed solvent of oxyethane (30 mL) and water (6 mL), the mixture was heated to reflux for 9 hours under a nitrogen atmosphere. After the reaction, the reaction solution was poured into saturated brine and extracted with ethyl acetate. After drying the obtained organic layer with anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure. The residue was purified by silica gel column...
Embodiment 3
[1087]Production of 4-(4-chloro-2-methoxyphenyl)-1-(pyridin-3-yl)-1H-pyrazole-3-carboxamide (the compound number of the present invention: A-0634)
[1088]1) 4-(4-Chloro-2-methoxyphenyl)-1-(pyridin-3-yl)-1H-pyrazole-3-carboxylic acid ethyl ester (the compound number of the present invention: C-0634)
[1089]The 4-{[(nonafluorobutyl)sulfonyl]oxy}-1-(pyridin-3-yl)-1H-pyrazole-3-carboxylic acid ethyl ester (0.42g, 0.82mmol), 4-chloro -2-Methoxyphenylboronic acid (0.20g, 1.1mmol), sodium carbonate (0.19g, 1.8mmol) and tetrakis (triphenylphosphine) palladium (0.050g, 0.043mmol) were added to tetrahydrofuran (5.0mL) and water (0.50 mL) in a mixed solvent and heated to reflux for 3 hours under a nitrogen atmosphere. After the reaction, the reaction solution was poured into saturated brine and extracted with ethyl acetate. After drying the obtained organic layer with anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure. The residue was purified by silica gel column ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com