Synthetic method of (s)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamine
A technology of methoxyphenyl and methanesulfonyl, which is applied in the field of synthesis of -1--2-ethylamine, can solve the problems of high synthesis cost and low yield of chiral amine intermediates, and achieve simple treatment of three wastes Easy to operate, easy to separate and purify, and environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] A synthetic method of (S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamine, comprising the steps of:
[0038] Mix 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyleneamine, chiral catalyst, acid and solvent, and react;
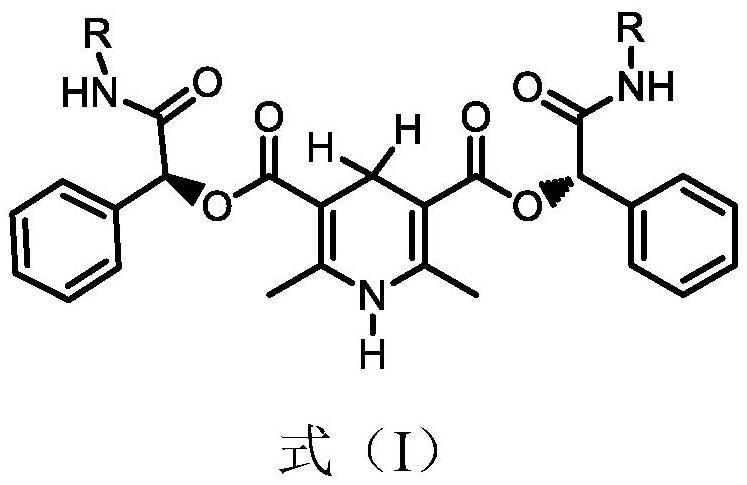
[0039] The structural formula of the chiral catalyst is shown in formula (I):
[0040]
[0041]R is selected from a hydrogen atom, a straight-chain alkyl group with 1-20 carbon atoms, a branched-chain alkyl group with 3-20 carbon atoms, a cycloalkyl group with 3-10 carbon atoms, a cycloalkyl group with 3-10 ring atoms Heterocyclyl, alkoxy with 1-20 carbon atoms, trifluoromethyl, halogen, amino, cyano, hydroxyl, nitro, ester, amido, substituted or unsubstituted with 6-20 rings Atomic aryl, substituted or unsubstituted heteroaryl having 5-20 ring atoms.
[0042] The invention provides a synthetic method of (S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamine, which uses 1-(3-ethoxy Base-4-methoxyphenyl)-2-(methylsulfonyl)vinylam...
Embodiment 1
[0078] This example provides a synthesis method of (S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamine.
[0079] (1) Preparation of Chiral Catalyst I:
[0080] 2,2,6-Trimethyl-1,3-dioxin-4-one (142.2 mg, 1 mmol) was added dropwise to (S)-α-hydroxy-N-methyl-2-phenylacetamide ( 165.1mg, 1mmol) in toluene (0.5mL) solution. After stirring at reflux overnight, the reaction mixture was cooled to 50°C and the solvent was removed in vacuo. The crude product was purified by silica gel column chromatography (the volume ratio of n-hexane:ethyl acetate was 20:1) to obtain 187.0 mg of white solid compound with a yield of 75%.
[0081] The above white solid compound (498.6mg, 2mmol), ammonium acetate (77.1mg, 1mmol) and hexamethylenetetramine (140.2mg) were dissolved in 5mL of dioxane, and heated at 100°C for 30 minutes. Cool to room temperature, add water, extract with dichloromethane, and collect the organic phase. After the organic phase was concentrated, it was purified by...
Embodiment 2
[0086]In this embodiment, a synthetic method of (S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamine, the steps are the same as in Example 1, the difference is that: The chiral catalyst for is Catalyst II.
[0087] (1) Preparation of Chiral Catalyst II:
[0088] 2,2,6-Trimethyl-1,3-dioxin-4-one (142.2 mg, 1 mmol) was added dropwise to (S)-α-hydroxy-N-ethyl-2-phenylacetamide ( 179.2mg, 1mmol) in toluene (0.5mL) solution. After stirring at reflux overnight, the reaction mixture was cooled to 50°C and the solvent was removed in vacuo. The crude product was purified by silica gel column chromatography (the volume ratio of n-hexane:ethyl acetate was 20:1) to obtain 243.0 mg of white solid compound with a yield of 82%.
[0089] The above white solid compound (526.6mg, 2mmol), ammonium acetate (77.1mg, 1mmol) and hexamethylenetetramine (140.2mg) were dissolved in 5mL of dioxane, and heated at 100°C for 30 minutes. Cool to room temperature, add water, extract with dichlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com