Compound for detecting tyrosinase and application thereof
A technology of tyrosinase and compounds, applied in the field of fluorescent probes, can solve the problems of poor biocompatibility and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Synthesis of FluotyLu1
[0046]
[0047] Compound 4 (154 mg, 0.748 mmol) and 6-amino-2-cyanobenzothiazole (compound 7, 131 mg, 0.748 mmol) were dissolved in MeCN (8 ml). AcOH (400 μl) and NaBH were added sequentially 3 CN (100 mg, 1.59 mmol). The mixture was stirred at room temperature. Add NaHCO after 10 minutes 3 Aqueous solution (30ml), all extracted with ethyl acetate (200ml). Combine the organic layers with H 2 O and brine wash. The organic layer was washed with Na 2 SO 4 Dry and evaporate the solvent. The residue was purified by flash silica gel column chromatography with a mixed solvent of ethyl acetate and n-hexane to obtain compound 9.
[0048] 1 H NMR (500MHz, CDCl 3 )δ=7.88(d,1H),6.84-6.80(m,2H),6.64(d,1H),6.59-6.57(m,2H),4.151(s,1H,NH),,3.19(t,2H) ), 2.65(t, 2H), 1.94(quintet, 2H), 1.66(s, 6H).
[0049] 13CNMR (500MHz, DMSO-d6), δ=150.51, 147.22, 145.37, 139.35, 135.30, 128.02, 125.23, 120.98, 117.94, 117.75, 114.86, 109.01, 108.28...
Embodiment 2
[0055] Example 2 Synthesis of FluotyLu 2
[0056]
[0057] 2-Cyano-6-hydroxybenzothiazole (compound 6, commercially available, 150 mg, 0.85 mmol) was placed in DMF and K as the base 2 CO 3 (352.4 mg, 2.55 mol) was added to the reaction solution. After the mixture was stirred at room temperature for 15 minutes, compound 11 (1.15 g, 4.26 mmol) was added, and the temperature of the mixture was heated to 70°C overnight. When the reaction was completed under TLC monitoring, the reaction mixture was cooled, diluted with ethyl acetate, and washed 3 times with saturated brine. The organic phase was dried over anhydrous magnesium sulfate, then the solvent was removed under reduced pressure, and the crude solid product was purified by flash silica gel column chromatography with a mixed solvent of ethyl acetate and petroleum ether to obtain compound 12.
[0058] Compound 12 (0.10 mmol) was added to 10 ml of degassed CH in a 50 ml round bottom flask equipped with a stir bar under ar...
Embodiment 3
[0059] Example 3 Performance of FluotyLu1
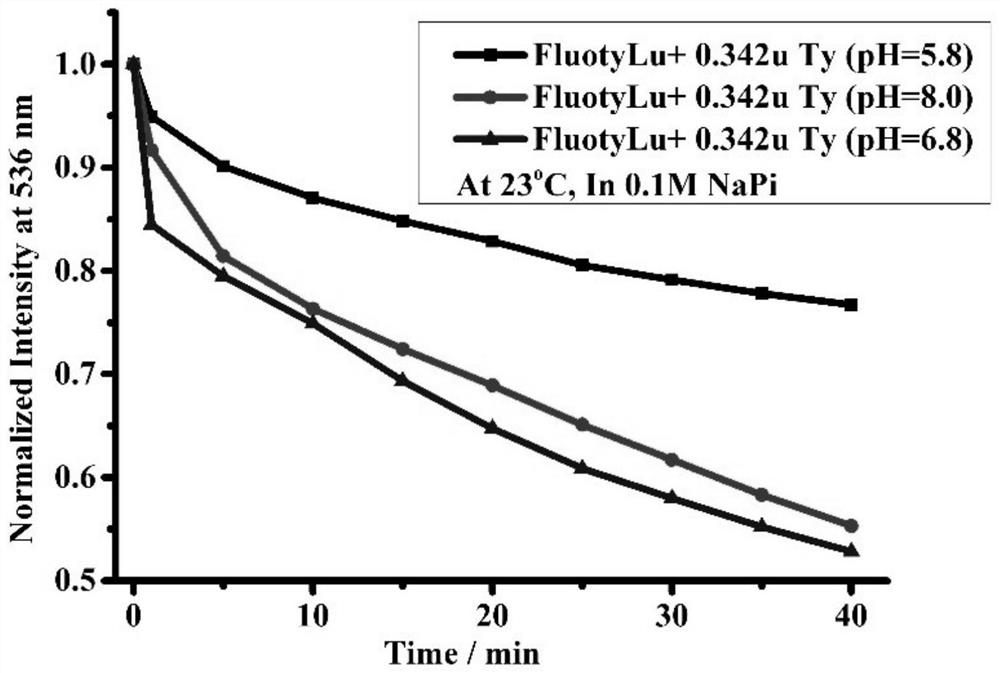
[0060] figure 1 The time-dependent fluorescence spectra of FluotyLu1 solutions at different pH values for Tyr detection activity were determined. The results showed that the detection activity of FluotyLu1 was severely inhibited under the condition of pH=5.8; the detection was slightly inhibited under the condition of pH=8.0, and the detection activity of FluotyLu1 in PBS with pH value of 6.8 was exhibited.
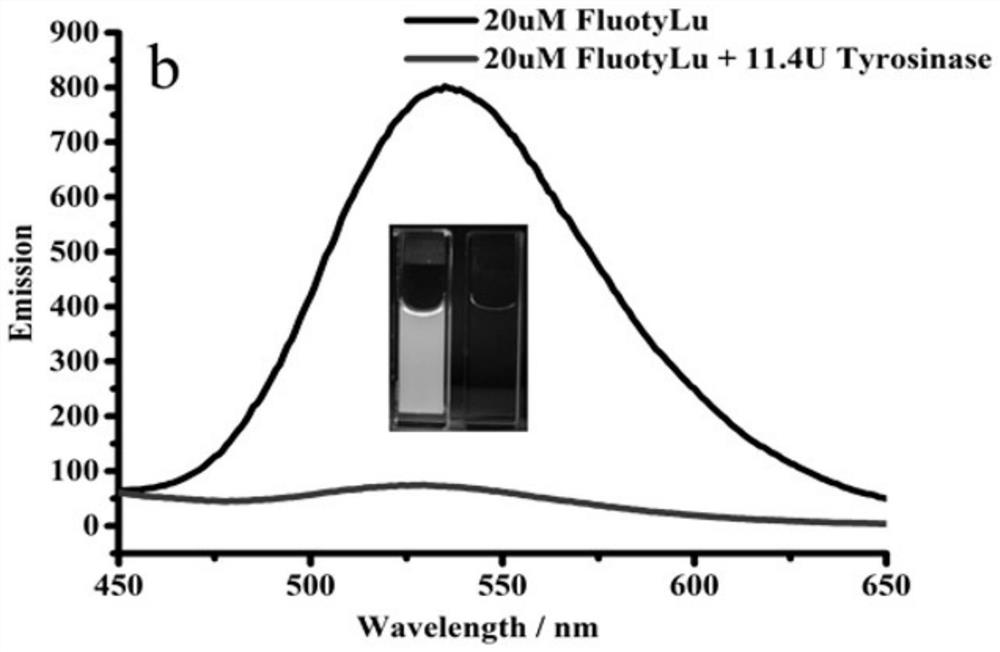
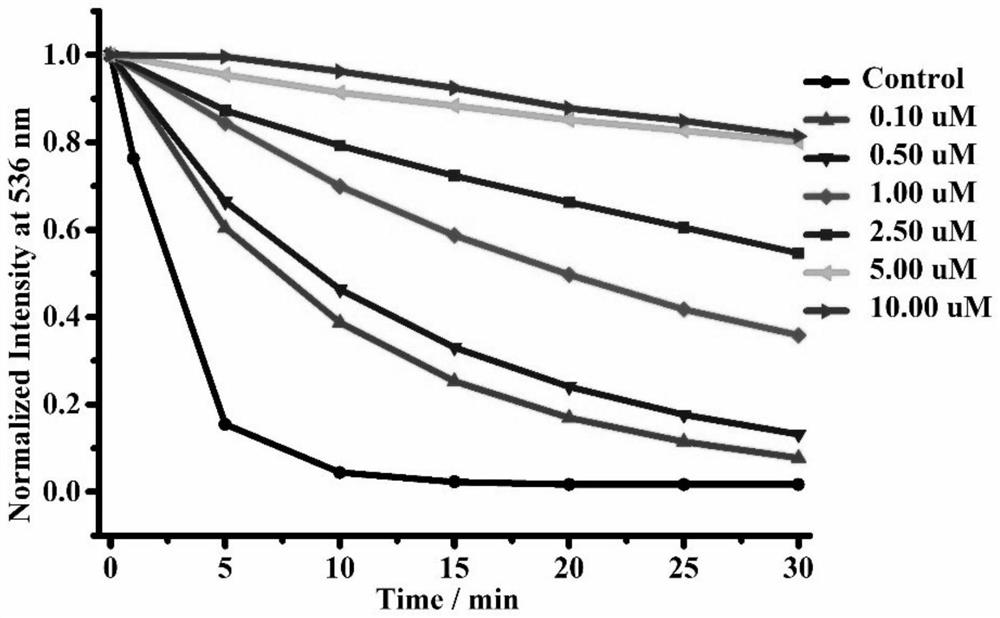
[0061] figure 2 It was determined that the maximum emission peak of FluotyLu1 was located at 535nm, showing a characteristic green fluorescence (the left cuvette in the fluorescence snapshot); after the addition of Tyr, its fluorescence decreased rapidly within 2min and finally disappeared (the right color in the fluorescence snapshot). dish). It can be speculated that there is a photoinduced electron transfer (PET) process between the ortho-diphenol hydroxyl group (electron donating group) and the fluorescein structure. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com