Preparation method of high-purity esketamine hydrochloride ketone body
A technology for esketamine hydrochloride ketone body and acyl chloride, which is applied in the field of preparation of high-purity esketamine hydrochloride ketone body, can solve problems such as low yield, long reaction time, and high toxicity, and achieve high yield and reaction Effects in short duration and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
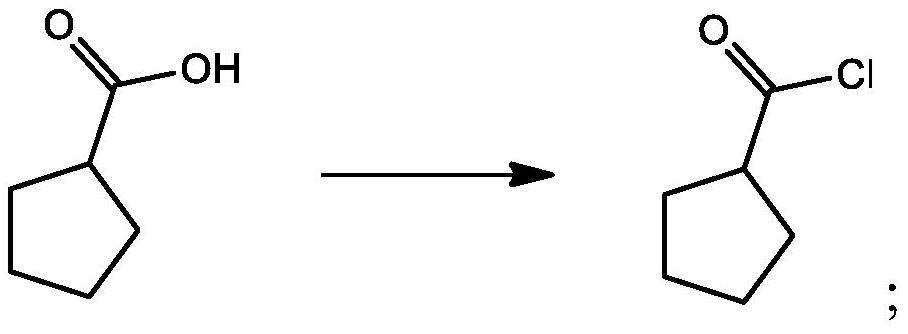
[0060] Embodiment 1 - the preparation of cyclopentacarbonyl chloride
[0061] Add 262.7g of thionyl chloride into the reaction flask, stir, and add 210g of cyclopentanoic acid dropwise at 40-50°C. After the dropwise addition, keep warm at 40-50°C for 2 hours. After treatment, concentrated under reduced pressure to remove excess thionyl chloride to obtain 237.5 g of cyclopentanoyl chloride with a yield of 97.4%.
Embodiment 2
[0062] Embodiment 2 - the preparation of o-chlorophenyl magnesium chloride
[0063] Under the protection of nitrogen, add 1306ml tetrahydrofuran solution of isopropylmagnesium chloride and lithium chloride with a concentration of 1.3mol / L into the reaction bottle, stir, cool down to 0-10°C, add 250g of o-chlorobromobenzene dropwise, after the dropwise addition, keep the temperature React at 0-10°C for 4 hours and set aside.
Embodiment 3
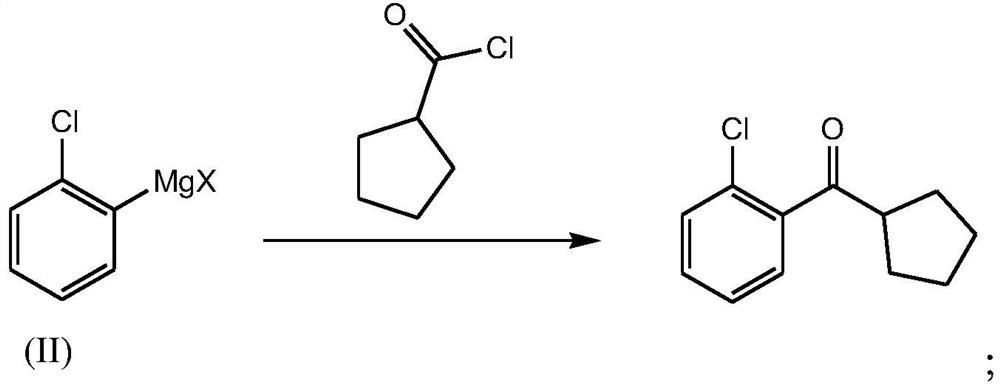
[0064] Embodiment 3 - the preparation of o-chlorophenyl cyclopentyl ketone
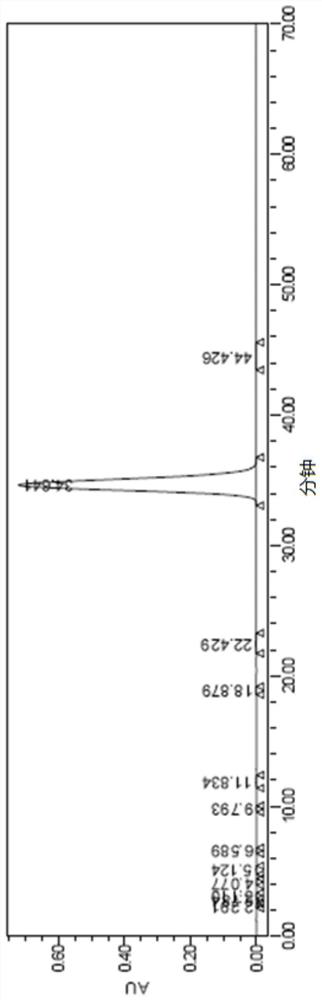
[0065] Under the protection of nitrogen, stir, and cool down the reaction solution in Example 2 to -10~0°C, add 225.1 g of cyclopentanoyl chloride in Example 1 dropwise, after the dropwise addition, keep warm at -10~0°C for reaction 2 Hours, after treatment, add 450ml of 2mol / L dilute hydrochloric acid dropwise, divide and remove the lower layer, wash the upper organic layer with 13% aqueous sodium chloride solution, then wash with 10% sodium hydroxide, wash with saturated sodium chloride, and concentrate under reduced pressure to remove the low boiling point solvent, and then distilled to obtain 208 g of o-chlorophenyl cyclopentyl ketone, with a yield of 76.3% and a purity of 98.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com