Electrochemical synthesis method of 1,3-dimethyl-3-difluoroethyl-2-oxindole compound
A technology of indole compound and difluoroethyl, applied in the field of electrochemical synthesis of 1,3-dimethyl-3-difluoroethyl-2-oxindole compound, achieving high yield and mild reaction conditions , the effect of wide selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
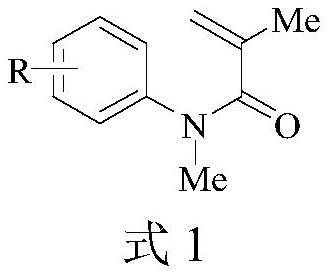
[0046]The following Examples 1 to 5 are all reacted according to the following reaction equations, mainly to investigate the yields of different substrates under optimal conditions:
[0047]
[0048]The specific operation steps are as follows: in a 25mL three-necked round bottom flask, add N-methyl-N-arylacryloyl (0.5mmol), difluoroacetic acid (1mmol), potassium iodide (0.025mmol), HFIP (8mL), water in sequence (0.2mL), 15mm×15mm×3mm foamed copper electrode as anode, 15mm×15mm×3mm foamed zinc electrode as cathode. The resulting mixed solution was stirred and reacted for 12 hours under a DC current of 15 mA at room temperature. The thin-layer chromatography plate tracks the reaction process. After the reaction is completed, the extract is concentrated by a rotary evaporator, using petroleum ether / ethyl acetate as the eluent, and silica gel for column chromatography purification.
Embodiment 1
[0050]The yield is 95%,
[0051]5-chloro-3-(2,2-difluoroethyl)-1,3-dimethylindolin-2-one;
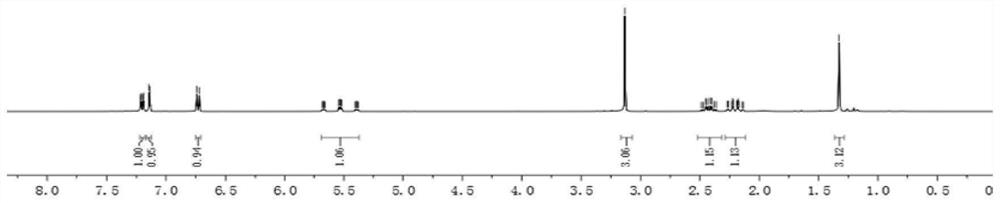
[0052]1H NMR(400MHz, CDCl3): δ7.23-7.19(m,1H), 7.14(d,J=2.0Hz,1H), 6.72(d, J=8.0Hz,1H), 5.57(tdd,J=56.0,6.4,3.6Hz, 1H), 3.13(s, 3H), 2.49-2.35(m, 1 H), 2.27-2.13(m, 1H), 1.33(s, 3H);
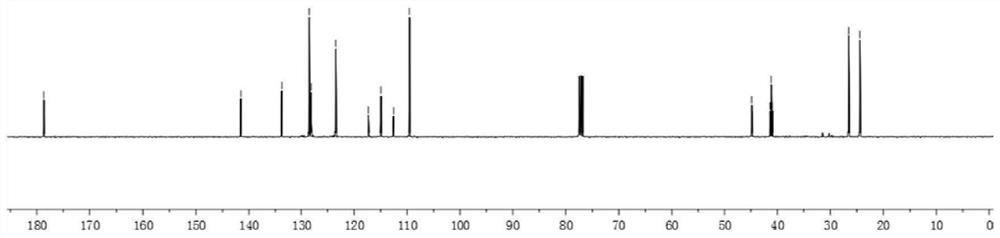
[0053]13C NMR(100MHz, CDCl3): δ178.7, 141.5, 133.8, 128.5, 128.2, 123.5, 114.9 (t, J = 238.5 Hz), 109.6, 44.8 (t, J = 5.2 Hz), 41.2 (t, J = 21.8 Hz), 26.5, 24.4.
Embodiment 2
[0055]Yield 88%
[0056]3-(2,2-difluoroethyl)-1,3,6-trimethylindolin-2-one;
[0057]1H NMR(400MHz, CDCl3): δ7.19(t,J=7.6Hz,1H), 6.89(d,J=8.0Hz,1H), 6.71(d,J=8.0Hz,1H), 7.85(s,1H), 6.86(d ,J=8.0Hz,1H),5.55(tdd,J=56.0, 6.4,3.2Hz,1H), 5.47(t,J=56.0,1H), 3.20(s,3H), 2.69–2.57(m,1H ),2.54– 2.47(m,1H),2.37(s,3H);
[0058]13C NMR(100MHz, CDCl3): δ179.2,143.2,134.3,128.9,125.3,122.5,115.3(t,J=238.2Hz),106.3,44.4(dd,J=4.2Hz,6.4Hz),39.9,26.3,21.8,18.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com