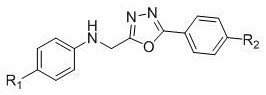
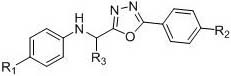
Method for synthesizing heteroaryl methylamine compound through oxidation reduction-decarboxylation coupled reaction
A heteroaryl methylamine, decarboxylation coupling technology, applied in the direction of organic chemistry, etc., to achieve the effect of low production cost, convenient operation, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Synthesis of N-(cyclohexyl(5-phenyl-1,3,4-oxadiazol-2-yl)methyl)-4-methylaniline
[0031]
[0032] Add magnetons to a dry 10mL reaction tube, followed by N-((5-phenyl-1,3,4-oxadiazol-2-yl)methyl)4-methylaniline (0.2mmol) And cyclohexane NHP ester (0.3mmol), copper acetylacetonate (10 mol%, 0.02mmol), 3,4,7,8-tetramethyl-1,10-phenanthroline (15 mol%, 0.03mmol) , (9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphine) (15 mol%, 0.03mmol) and triethylenediamine (0.4mmol, 2.0 equiv) , then pump out the air and backfill with Ar (3 times). Acetonitrile (2 mL) was added, the reaction tube was transferred to a blue light reactor (6 W) and irradiated there for 12 h. The solvent was distilled off under reduced pressure and separated by column chromatography (silica gel: 200-300 mesh, eluent volume ratio: n-hexane:ethyl acetate=15:1). The solvent was distilled off under reduced pressure to obtain a white solid product, namely N-(cyclohexyl(5-phenyl-1,3,4-oxadiazol...
Embodiment 2
[0034] Example 2: Synthesis of N-(cyclohexyl(5-phenyl-1,3,4-oxadiazol-2-yl)methyl)-4-methoxyaniline:
[0035]
[0036] Add magnetons to a dry 10mL reaction tube, followed by adding N-((5-phenyl-1,3,4-oxadiazol-2-yl)methyl)4-methoxyaniline (0.2mmol ) and cyclohexane NHP ester (0.3mmol), copper acetylacetonate (10 mol%, 0.02mmol), 3,4,7,8-tetramethyl-1,10-phenanthroline (15 mol%, 0.03mmol ), (9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphine) (15 mol%, 0.03mmol) and triethylenediamine (0.4mmol, 2.0 equiv ), then evacuated and backfilled with Ar (3 times). Acetonitrile (2 mL) was added, the reaction tube was transferred to a blue light reactor (6 W) and irradiated there for 12 h. The solvent was distilled off under reduced pressure and separated by column chromatography (silica gel: 200-300 mesh, eluent volume ratio: n-hexane:ethyl acetate=15:1). The solvent was distilled off under reduced pressure to obtain a white solid product, namely N-(cyclohexyl(5-phenyl-1,3,4-o...
Embodiment 3
[0038] Embodiment 3: the synthesis of N-(benzo[d]oxazol-2-yl(cyclohexyl)methyl)-4-methylaniline
[0039]
[0040] Add magnetons to a dry 10mL reaction tube, followed by sequential addition of N-(benzo[d]oxazol-2-ylmethyl)-4-methylaniline (0.2mmol) and cyclohexane NHP ester (0.3 mmol), copper acetylacetonate (10 mol%, 0.02mmol), 3,4,7,8-tetramethyl-1,10-phenanthroline (15 mol%, 0.03mmol), (9,9-dimethyl yl-9H-xanthene-4,5-diyl)bis(diphenylphosphine) (15 mol%, 0.03 mmol) and triethylenediamine (0.4 mmol, 2.0 equiv), then evacuate the air and backfill with Ar (3 times). Acetonitrile (2 mL) was added, the reaction tube was transferred to a blue light reactor (6 W) and irradiated there for 12 h. The solvent was distilled off under reduced pressure and separated by column chromatography (silica gel: 200-300 mesh, eluent volume ratio: n-hexane:ethyl acetate=15:1). The solvent was distilled off under reduced pressure to obtain a white solid product, namely N-(benzo[d]oxazol-2-yl(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com