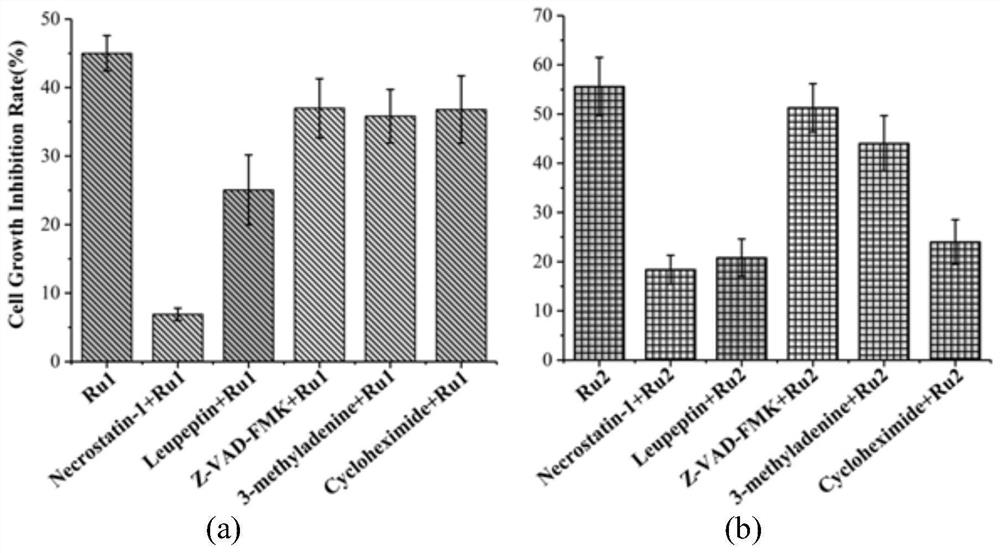
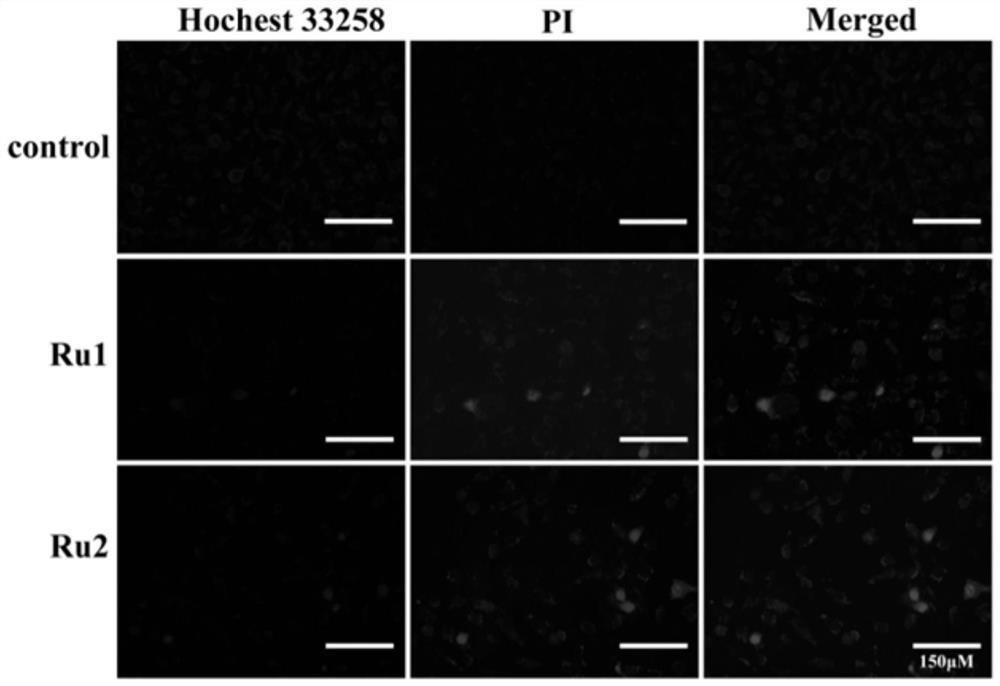
Ursolic acid piperazine dithioformic acid-pyridine ruthenium complexes as well as preparation method and application thereof
A technology of ursolic acid piperazine dithiocarboxylic acid and dithiocarboxylic acid, which is applied in the directions of ruthenium organic compounds, pharmaceutical formulations, steroids, etc. The preparation method of ruthenium complexes has the problems of cytotoxicity and other problems, and achieves the effects of significant inhibitory activity, simple preparation method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of ligand L
[0039] Get compound UAP (5.24g, 10mmol, 1.0equiv), 4mmol / L sodium hydroxide (2.5mL, 10mmol, 1.0equiv) and carbon disulfide (5mL) and place in the round bottom flask, after adding 100mL of methylene chloride (at this time the system pH>9), stirred the reaction at room temperature for 4h, stopped the reaction, evaporated the solvent, and purified the residue by silica gel column chromatography (dichloromethane / methanol=250:3, volume ratio) to obtain 4.53g of white powdery solid , yield 72.8%. HRMS(m / z)(ESI):C 35 h 55 N 2 NaO 2 S 2 [L+Na] + calcd for: 645.3500, found: 645.3601; C 35 h 55 N 2 NaO 2 S 2 [L-Na] - calcd for:599.3705,found:599.3714. 1 H NMR (500MHz, DMSO-d 6 )δ5.30(s,1H,OH),5.09(s,1H,12-H),4.35–3.51(br,9H,3-H and 4×CH 2 in piperazine),3.04–2.94(br,1H),2.35(d,J=10.5Hz,1H),2.00(dd,J=69.7,59.1Hz,6H),1.57–1.13(m,14H),1.03( s,3H,27-CH 3 ), 0.92 (d, J=5.1Hz, 4H, 26-CH 3 and CH),0.88(s,3H,25-CH 3 ),0.86–...
Embodiment 2
[0042] Embodiment 2: Preparation of Ligand L
[0043] Get compound UAP (2.62g, 5mmol, 1.0equiv), 4mmol / L sodium hydroxide (1.25mL, 5mmol, 1.0equiv) and carbon disulfide (4mL) are placed in the round bottom flask, after adding 50mL ethanol (the pH of this moment system >9), stirred the reaction at room temperature for 2h, stopped the reaction, evaporated the solvent, and purified the residue by silica gel column chromatography (dichloromethane / methanol=250:3, volume ratio) to obtain 1.82g of white powdery solid, product The rate is 58.4%.
[0044] The structure of the product obtained in this example was characterized by high-resolution mass spectrometry, proton nuclear magnetic resonance spectrum, and carbon nuclear magnetic resonance spectrum, and it was determined that the product obtained in this example was ligand L.
Embodiment 3
[0045] Embodiment 3: Preparation of Ligand L
[0046] Example 2 was repeated, except that methanol, tetrahydrofuran, dioxane, chloroform or cyclohexane were used instead of ethanol, and potassium carbonate was used to adjust the pH of the system to 10. Finally, a colored powdery solid was obtained. The products obtained from the reaction in different solvents were characterized by high-resolution mass spectrometry, proton nuclear magnetic resonance spectrum, carbon nuclear magnetic resonance spectrum, etc., and it was confirmed that the obtained products were all ligand L.
[0047] The following examples prepare complex I, complex II and complex III according to the following synthetic routes:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com