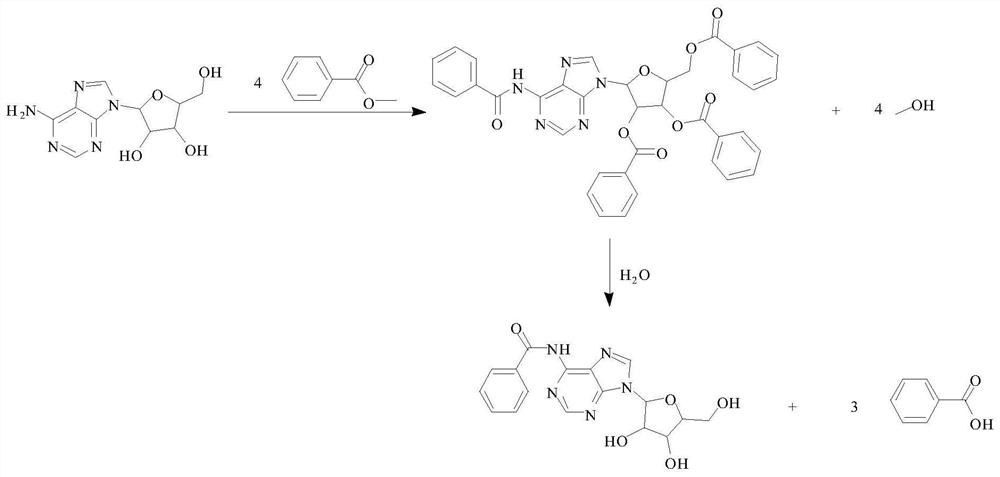
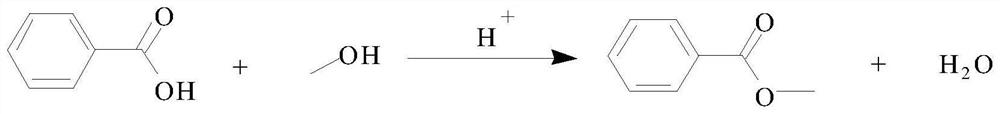
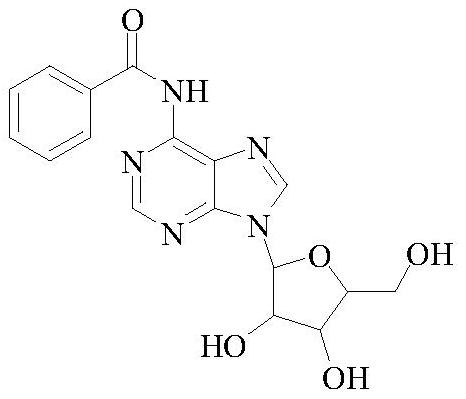
Method for preparing N6-benzoyladenosine
A technology for benzoyladenosine and adenosine, which is applied in the field of preparation of N6-benzoyladenosine, can solve problems such as serious environmental pollution and cannot meet environmental protection requirements, achieve environmental friendliness, improve enterprise production efficiency, and reduce costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Weigh 100.0g of adenosine and 240.0g of methyl benzoate into a dry four-necked flask, add 800.0g of toluene and 4.0g of p-trifluoroacetic acid, stir, reflux for 2 hours, distill 100ml of solvent, and continue to keep warm 2h;
[0028] (2) Cool the mixture of the above step (1) to 5°C, stir for 0.5h, filter, rinse with 200ml toluene once, and the filter cake is esterified benzoyladenosine;
[0029] (3) Add the filter cake obtained in the above step (2) into a four-necked bottle, add 600g of deionized water and 100g of sodium bicarbonate, heat up to 40°C, stir for 0.5h, filter, and rinse the filter cake with deionized water three times (200ml × 3), dried to obtain N6-benzoyladenosine 93.3g, purity 99.5%, yield 93.3%; the nuclear magnetic spectrum data of product N6-benzoyladenosine are as follows: 1 H NMR (600MHz, DMSO-d 6 )δ11.21 (s, 1H), 8.74 (d, J=23.9Hz, 2H), 8.05 (d, J=7.6Hz, 2H), 7.65 (t, J=7.4Hz, 1H), 7.56 (t, J=7.6Hz, 2H), 6.04(d, J=5.7Hz, 1H), 5.25(d, J=4....
Embodiment 2
[0032] (1) Weigh 100.0g of adenosine and 250.0g of methyl benzoate into a dry four-neck flask, add 1000.0g of toluene and 4.0g of p-trifluoroacetic acid, stir, reflux for 4 hours, distill 200ml of solvent, and continue to keep warm 3h;
[0033] (2) Cool the mixture of the above step (1) to 0° C., stir for 0.5 h, filter, rinse once with 200 ml of toluene, and the filter cake is esterified benzoyladenosine;
[0034] (3) Add the filter cake obtained in the above step (2) into a four-necked bottle, add 600g of deionized water and 100g of sodium bicarbonate, heat up to 30°C, stir for 0.5h, filter, and rinse the filter cake with deionized water three times (200ml×3), dried to obtain N6-benzoyladenosine 94.0g, purity 99.7%, yield 94.0%;
[0035] (4) the filtrate of above-mentioned step (2) is carried out rectifying, isolate the methanol 29.8g wherein, the filtrate distillation of step (3) is separated benzoic acid 114.0g, methyl alcohol and benzoic acid are esterified under sulfuric...
Embodiment 3
[0037] (1) Weigh 20.0g of adenosine and 46.0g of ethyl benzoate into a dry four-neck flask, add 140.0g of benzene and 0.6g of p-toluenesulfonic acid, stir, reflux for 6 hours, distill 20ml of solvent, and continue to keep warm 4h;
[0038] (2) Cool the mixture of the above step (1) to 10° C., stir for 0.5 h, filter, rinse once with 40 ml of benzene, and the filter cake is esterified benzoyladenosine;
[0039] (3) Add the filter cake obtained in the above step (2) into a four-necked bottle, add 120.0g deionized water and 13g sodium carbonate, heat up to 50°C, stir for 0.5h and then filter, and rinse the filter cake with deionized water three times (20ml×3), dried to obtain N6-benzoyladenosine 18.4g, purity 99.6%, yield 92.0%;
[0040] (4) the filtrate of above-mentioned step (2) is carried out rectifying, isolate ethanol 13g wherein, the filtrate distillation of step (3) is separated to go out benzoic acid 34g, and ethanol and benzoic acid are esterified under sulfuric acid ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com