Fluorescence-radioactivity combined in-vitro targeted screening method
A screening method and radioactive technology, applied in the field of fluorescence-radioactive combined in vitro targeted screening, can solve the problems of high hardware requirements, inconsistent sensitivity and radioactive signals, difficult and high-throughput rapid screening research, and achieve simple operation. , the effect of reducing the probability of false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
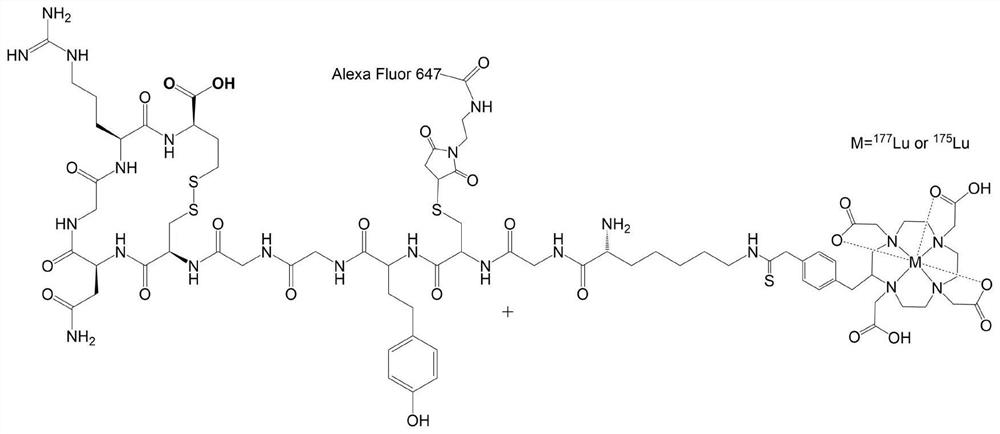
[0029] In this embodiment, a radioactive 177 Lu-DOTA-NGR polypeptide and its stable isotope-labeled 175 The preparation method of Lu-DOTA-NGR polypeptide, the specific steps are as follows:
[0030] Take 2 μl of DOTA-NGR peptide solution dissolved in sodium acetate buffer (0.25M, pH 5.5), add 20 μl of sodium acetate buffer (0.25M, pH 5.5) to dilute to a final concentration of 0.2 μmol / ml; add 177 Lu radionuclide 1mCi (specific activity: 800mCi / ml), heating reaction in 80℃ metal bath for 60min to obtain radionuclide-labeled target targeting molecule 177 Lu-DOTA-NGR polypeptide. The preparation steps of stable isotope-labeled target molecules are the same as above, except that the radionuclide 177 Lu is replaced by the corresponding stable isotope 175 Lu, that is 175 For Lu-DOTA-NGR polypeptide, the molar ratio of stable isotope and target targeting molecule is 1:10. The reaction was purified by HPLC to >95% radiochemical purity and >95% chemical purity. Radiolabeling was...
Embodiment 2
[0032] In this embodiment, a fluorescent probe Alexa Fluor 647maleimide label is provided 175 The preparation method of Lu-DOTA-NGR polypeptide, the specific steps are as follows:
[0033] Take the one in Example 1 175 Lu-DOTA-NGR polypeptide was dissolved in 20μl phosphate buffer (10mM, pH 7.4) to make the final concentration 0.2μmol / ml, add 1.5μg TCEP and 2μg maleimide fluorescent probe, react at room temperature for 4h, HPLC Purify and separate to obtain target targeting molecules with stable isotopes labeled with fluorescent probes, 175 Lu-DOTA-NGR-AlexaFluor647.
Embodiment 3
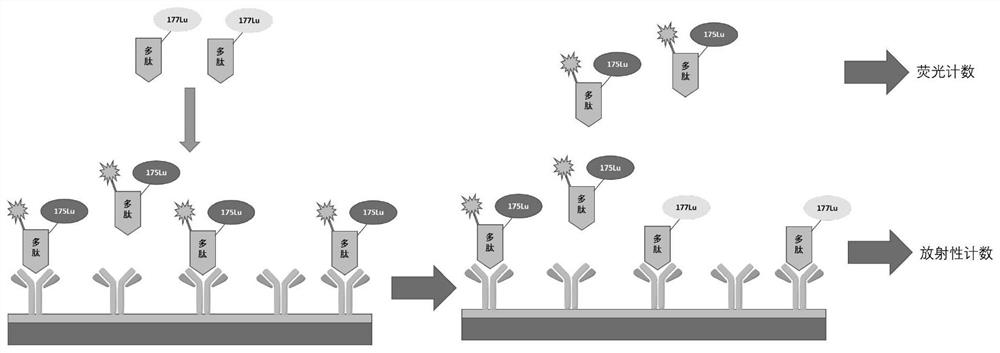
[0035] In this example, a specific binding 177 The method for screening monoclonal antibody drugs of Lu-DOTA-NGR target polypeptide molecules, the steps are as follows:
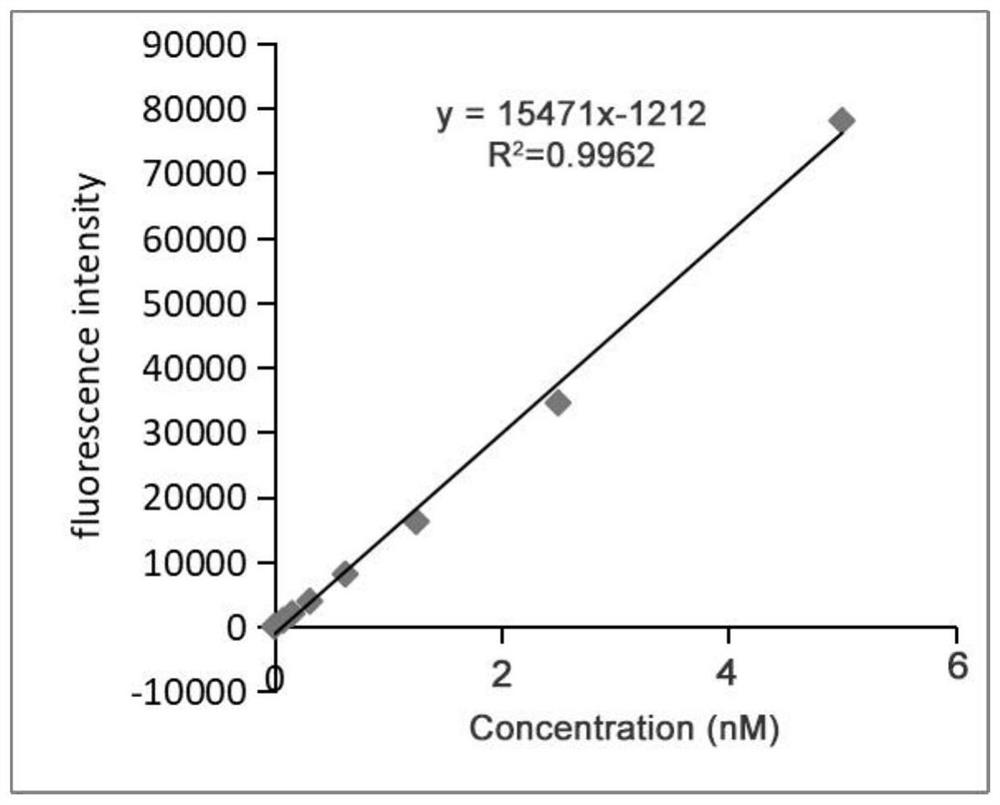
[0036]Take 10 monoclonal antibodies to be tested and dissolve them in phosphate buffer (10mM, pH 7.4), the final concentration of each monoclonal antibody is 5μg / ml, add 100μl of each antibody to a 96-well white plate, and incubate overnight at 4°C , each well was washed 3 times with 200 μl of phosphate buffer (10 mM, pH 7.4) containing 1% Tween-20; 200 μl of 3% bovine serum albumin solution (3g of bovine serum albumin dissolved in 100ml of phosphate buffer) was added to each well (10mM, pH 7.4)), incubate at 37°C for 2h; add 25μl of the fluorescent probe-labeled target molecule in Example 2 to each well 175 Lu-DOTA-NGR-Alexa Fluor 647 and 25 μl of the radionuclide-labeled target molecule in Example 1 177 Lu-DOTA-NGR; where 175 The concentration of Lu-DOTA-NGR-Alexa Fluor 647 is 100ng / ml, 177 The amount o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com