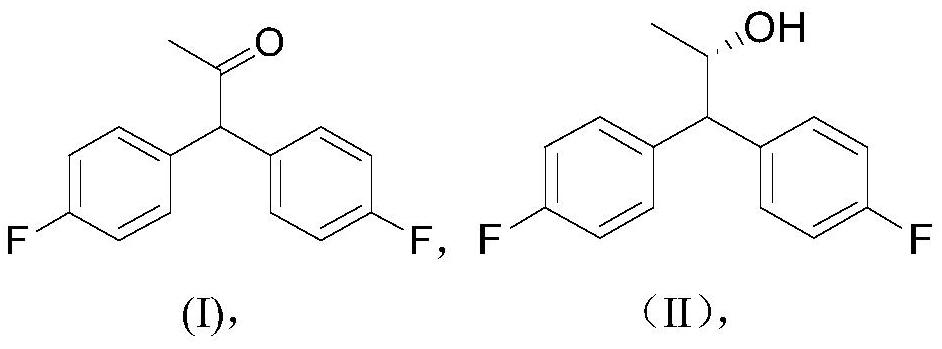
Preparation method of optically pure S-configuration 1, 1-bis-(4-fluorophenyl)-2-propanol
A technology of fluorophenyl and configuration, which is applied in the field of preparation of optically pure S-configuration 1,1-bis-2-propanol, which can solve problems such as yield reduction and product racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The present invention provides a method for preparing optically pure S-configuration 1,1-bis-(4-fluorophenyl)-2-propanol (II), which comprises, in an organic solvent, a compound represented by formula (I) in a catalyst and Asymmetric hydrogenation under alkaline conditions
[0018]
[0019] In some embodiments, the catalyst is a complex of a chiral ligand and a metal precursor, and the chiral ligand includes a tridentate ligand f-amphox, f-amphol, f-ampha, O-spiroPNN, Axial chiral bisphosphine ligands BINAP, SegPhos, MeO-Biphep, P-Phos, the metal precursors are ruthenium, rhodium, iridium salts, the structural formula of the chiral ligands is as follows:
[0020] In some embodiments, the molar ratio of the chiral ligand to the metal precursor is 1.0-1.5:1.
[0021] In some embodiments, the organic solvent is methanol, ethanol, isopropanol, tetrahydrofuran, toluene, 1,4-dioxane, methyl tert-butyl ether, dichloromethane, 1,2-dichloroethane Alkanes, ethyl acetate, n...
specific Embodiment approach
[0031] The reagents and raw materials used in the present invention are all available on the market.
[0032] The enantioselectivity of the present invention adopts following method to measure:
[0033] Chiracel AD-H, n-hexane / IPA=95:5, 1.0mL / min, 30℃, 230nm UV detector, t=11.72min for (S)isomer and t=13.54for (R)isomer
Embodiment 1
[0035] Add catalyst precursor [Ir(COD)Cl] to a 4.0 mL bottle under argon atmosphere 2 (6.71mg, 1.0×10 - 2 mmol, 1eq), ligand (f-amphox) (2.4×10 -2 mmol, 2.4eq) and anhydrous isopropanol ( i PrOH, 2.0 mL). The mixture was stirred at 25° C. for 12.0 h in an argon-filled glove box to obtain an orange-red solution, which can be directly used for catalytic reactions.
[0036] Add 246mg 1,1-bis-(4-fluorophenyl)-acetone (1mmol), 11.2mg potassium tert-butoxide to a glass test tube with a magnet, add 2ml of isopropanol under nitrogen protection, and add 10 μl 0.01M catalyst (S / C=10,000), filled with 4MPa hydrogen, reacted at 40°C for 24 hours. After the reaction, cool down to room temperature naturally, release the hydrogen carefully, filter with diatomaceous earth, and remove the organic solvent from the filtrate to obtain 241 mg of the product with a yield of 97% and an enantioselectivity of 80% ee.
[0037] colorless transparent liquid, 1 H NMR (400MHz, CDCl 3 )δ7.36–7.23(m,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com