Production process of high-purity aminoacetaldehyde dimethyl acetal
A technology of aminoacetaldehyde dimethyl acetal and chloroacetaldehyde dimethyl acetal, which is applied in the chemical industry, can solve environmental problems and energy consumption, increase the separation process, and difficult to separate finished products, etc., so as to reduce energy demand and improve reaction efficiency , The effect of increasing product output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
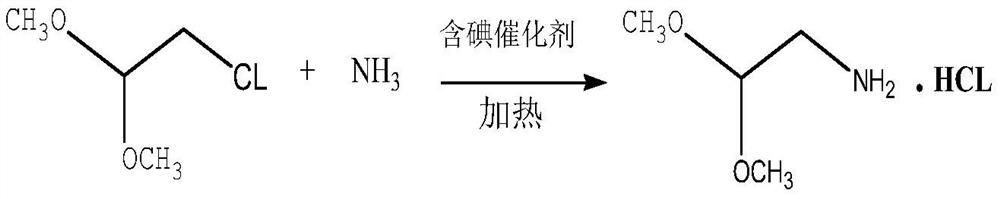
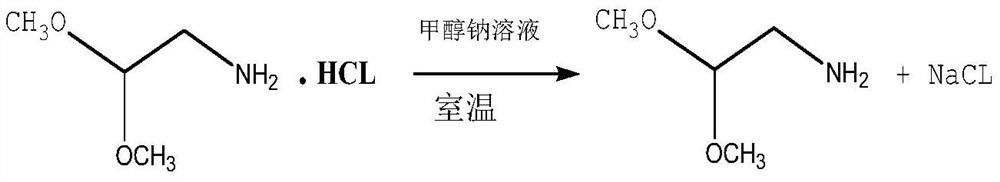
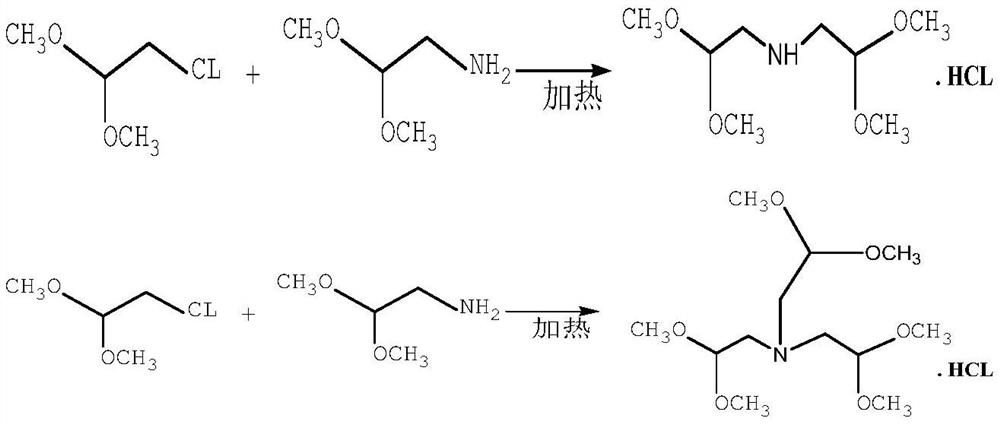
[0028] In a 3000L high-pressure reactor, use a metering pump to add chloroacetaldehyde dimethyl acetal to the kettle. The mass of chloroacetaldehyde dimethyl acetal is 250kg (2kmol), and then add 4.2kg of iodine and potassium iodide mixed catalyst (1:1) (0.02 kmol). After sealing, replace with argon three times, then add 1632kg of anhydrous liquid ammonia (120kmol) through the liquid ammonia metering pump, then start to heat up to 120°C, the pressure in the kettle reaches 9.0MPa, keep this temperature, and continue the reaction for 30 hours. After the reaction, the temperature is lowered to 60-70°C, ammonia is removed and ammonia is recovered until the temperature drops below 30°C, and the ammonia is recovered and stopped. Then add 360 kg (2 kmol) of sodium methoxide methanol solution with a mass fraction of 30% measured from the dropping tank, stir for 1 hour, and filter to remove salt. The solvent methanol was evaporated from the filtrate under normal pressure, and the crud...
Embodiment 2
[0030] In a 3000L high-pressure reactor, use a metering pump to add chloroacetaldehyde dimethyl acetal to the kettle. The mass of chloroacetaldehyde dimethyl acetal is 250kg (2kmol), and then add iodine and ammonium iodide mixed catalyst (1:1) 4.04kg (0.02kmol). After sealing, replace with argon three times, then add 1768kg of anhydrous liquid ammonia (130kmol) through the liquid ammonia metering pump, then start to heat up to 122°C, the pressure in the kettle reaches 9.3MPa, keep this temperature, and continue the reaction for 35 hours. After the reaction, the temperature is lowered to 60-70°C, ammonia is removed and ammonia is recovered until the temperature drops below 30°C, and the ammonia is recovered and stopped. Then add 360 kg (2 kmol) of sodium methoxide methanol solution with a mass fraction of 30% measured from the dropping tank, stir for 1 hour, and filter to remove salt. The solvent methanol was evaporated from the filtrate under normal pressure, and the crude pr...
Embodiment 3
[0032] In a 3000L high-pressure reactor, use a metering pump to add chloroacetaldehyde dimethyl acetal to the kettle. The mass of chloroacetaldehyde dimethyl acetal is 250kg (2kmol), and then add iodine and silver iodide mixed catalyst (1:1) 4.88kg (0.015 kmol). After sealing, replace with argon three times, then add 1768kg of anhydrous liquid ammonia (130kmol) through the liquid ammonia metering pump, then start to heat up to 122°C, the pressure inside the kettle reaches 9.3MPa, keep this temperature, and continue the reaction for 38 hours. After the reaction, the temperature is lowered to 60-70°C, ammonia is removed and ammonia is recovered until the temperature drops below 30°C, and the ammonia is recovered and stopped. Then add 360 kg (2 kmol) of sodium methoxide methanol solution with a mass fraction of 30% measured from the dropping tank, stir for 1 hour, and filter to remove salt. The solvent methanol was evaporated from the filtrate under normal pressure, and the crud...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com