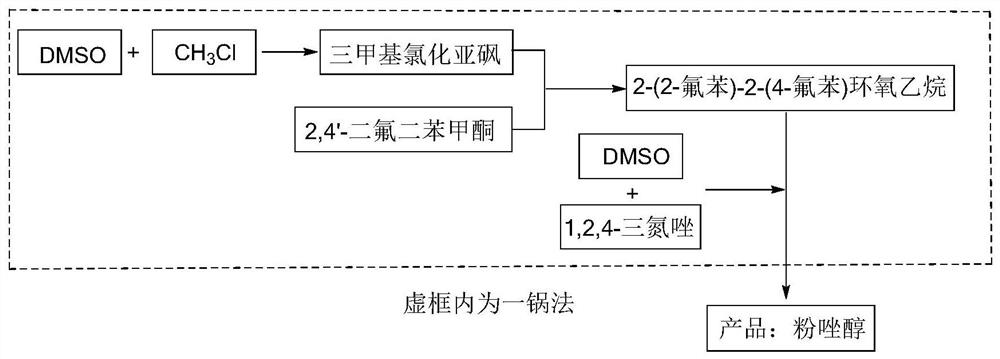
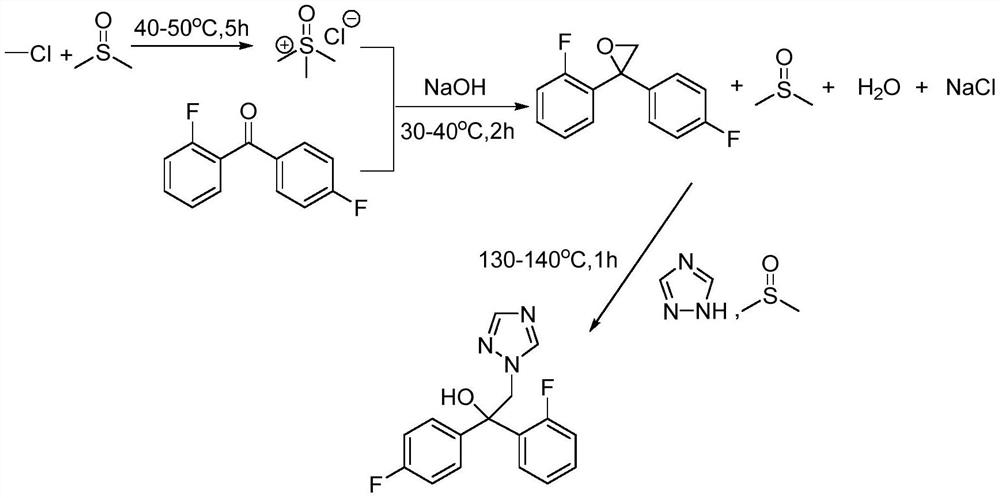
A kind of synthetic method of fuconazole
A synthetic method, the technology of triconazole, which is applied in the field of synthesis of triconazole, can solve problems such as a large amount of acid waste water and solid waste salt, limit the competitiveness of triconazole products, and affect the economic benefits of enterprises, and achieve high production capacity and responsiveness. The effect of short time and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 156g DMSO to the reactor, raise the temperature to 35-40°C, start to feed 50g of methyl chloride gas, the ventilation rate is not higher than 20g / h, the ventilation time is 2-3h, and keep warm at 35-40°C for 3h after the ventilation is completed. After the reaction, lower the temperature to 30-35°C and add 45g of sodium hydroxide in batches. After adding the sodium hydroxide, start to add 145g of 2,4'-difluorobenzophenone dropwise at a rate of 80g / h. The temperature is controlled at 30-40°C. After the dropwise addition, keep warm for 3 hours, then add 50 g of DMSO, add 55 g of triazole in batches, raise the temperature to 130-140° C., take samples for analysis after keeping warm for 2 hours, and the reaction is over. After the reaction, the temperature of the reactor was lowered to 50-60° C., the solid salt was filtered out by pressure filtration, and 30 g of DMSO was used to wash the solid salt. The filtered reaction solution was desolvated, and DMSO was recovered...
Embodiment 2
[0042] Add 156g DMSO to the reactor, raise the temperature to 35-40°C, start to feed 50g of methyl chloride gas, the ventilation rate is not higher than 20g / h, the ventilation time is 2-3h, and keep warm at 35-40°C for 3h after the ventilation is completed. After the reaction, lower the temperature to 30-35°C and add 45g of sodium hydroxide in batches. After adding the sodium hydroxide, start to add 145g of 2,4'-difluorobenzophenone dropwise at a rate of 80g / h. The temperature is controlled at 30-40°C. After the dropwise addition, keep warm for 3h, then add 250g of DMSO, add 55g of triazole in batches, raise the temperature to 130-140°C, keep warm for 2h, take samples for analysis, and the reaction is over. After the reaction, the temperature of the reactor was lowered to 50-60° C., the solid salt was filtered out by pressure filtration, and 30 g of DMSO was used to wash the solid salt. The filtered reaction solution was desolvated, and DMSO was recovered for reuse. Add 500g...
Embodiment 3
[0044]Add 156g DMSO to the reactor, raise the temperature to 35-40°C, start to feed 50g of methyl chloride gas, the ventilation rate is not higher than 20g / h, the ventilation time is 2-3h, and keep warm at 35-40°C for 3h after the ventilation is completed. After the reaction, lower the temperature to 30-35°C and add 35g of sodium hydroxide in batches. After adding the sodium hydroxide, start to add 145g of 2,4'-difluorobenzophenone dropwise at a rate of 80g / h. The temperature is controlled at 30-40°C. After the dropwise addition, keep warm for 3 hours, then add 10g of sodium hydroxide and 250g of DMSO, add 55g of triazole in batches, raise the temperature to 90-100°C, take samples for analysis after keeping warm for 7 hours, and the reaction is over. After the reaction, the temperature of the reactor was lowered to 50-60° C., the solid salt was filtered out by pressure filtration, and 30 g of DMSO was used to wash the solid salt. The filtered reaction solution was desolvated,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com