Novel coronavirus antibody detection kit as well as preparation method and application thereof
A coronavirus and kit technology, applied in the field of cell biology and immunoassay, can solve the problems of cumbersome operation, cumbersome steps and high sensitivity, and achieve the effects of high detection efficiency, simple operation and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1. Construction of novel coronavirus antibody detection kit
[0058] The magnetic bead fluorescent immunoassay kit for novel coronavirus antibody provided in this example has a specification of 100TEST and includes the following components:
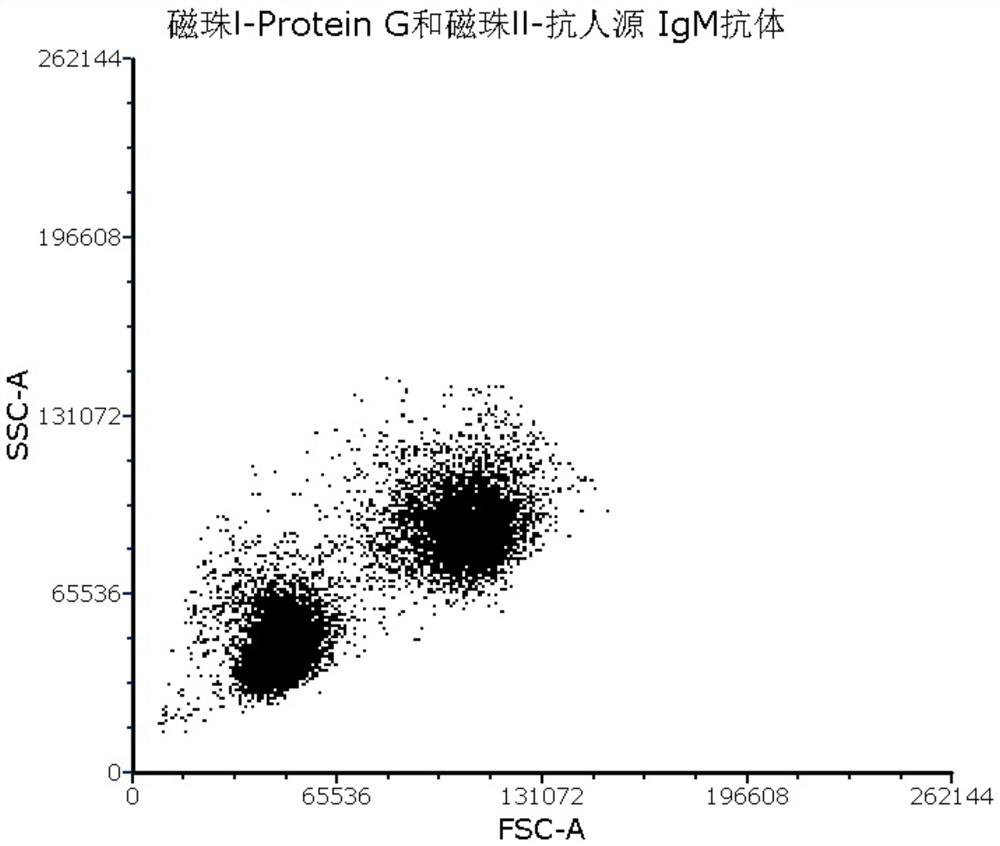
[0059] 1. Magnetic beads Ⅰ-Protein G, the specification is 1ml; magnetic beads I have a particle size of 2.8 μm, and are stored in a buffer, the buffer is 0.01M phosphate buffer containing 0.03% sodium azide, pH 7.4, the amount used 10μl / sample;
[0060]2. Magnetic beads II-anti-human IgM antibody, the specification is 1ml; the particle size of magnetic beads II is 10μm, and it is stored in buffer solution, the buffer solution is 0.01M phosphate buffer containing 0.03% sodium azide, pH7.4, The amount used is 10 μl / sample;
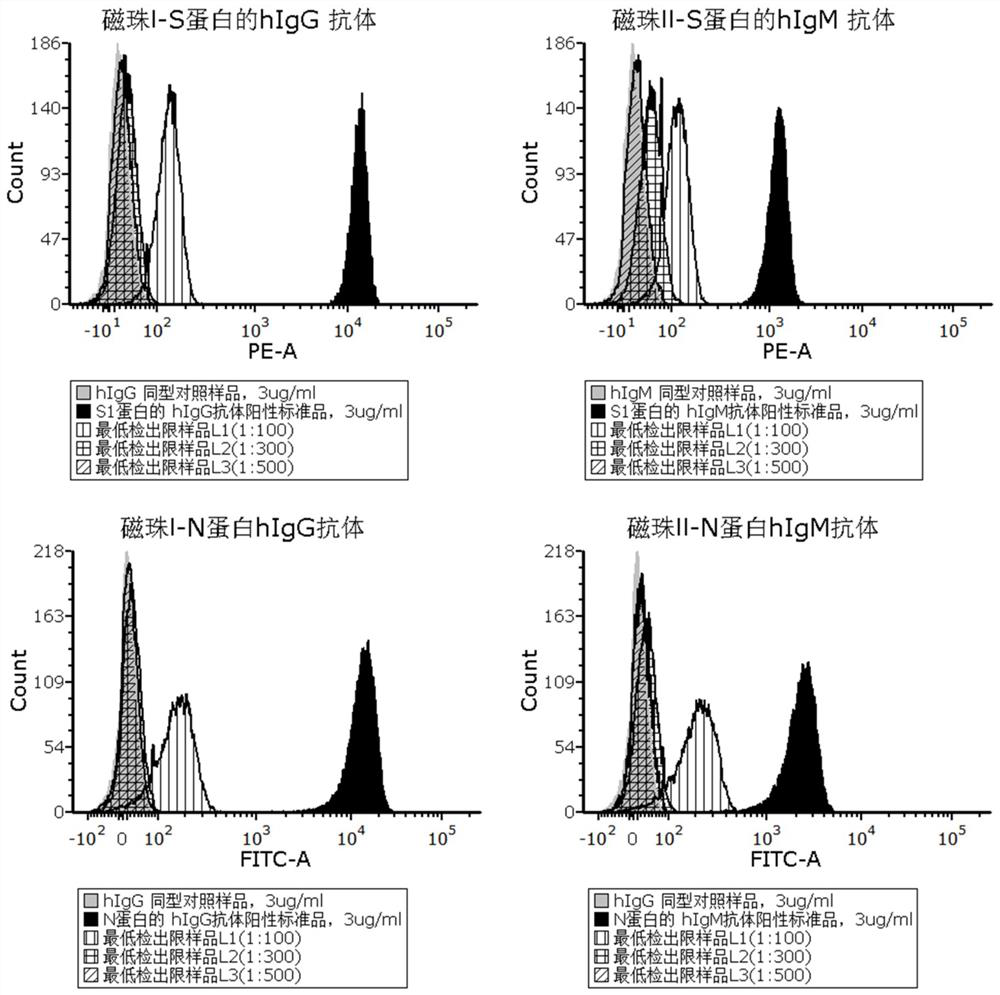
[0061] 3. PE-labeled novel coronavirus S1 protein, in the form of lyophilized powder, 1 vial, with a specification of 10 μg;
[0062] 4. FITC-labeled novel coronavirus N protein, in the form of freeze...
Embodiment 2
[0071] Embodiment 2, kit operation method
[0072] When the kit of Example 1 is used, the operation method is as follows:
[0073] 1. Take the PE-labeled novel coronavirus S1 protein, add 100 μl sterile ultrapure deionized water to reconstitute, the concentration is 100 μg / ml, use the flow detection buffer to prepare the working solution with a concentration of 2 μg / ml, and set aside.
[0074] 2. Take FITC-labeled novel coronavirus N protein, add 100 μl sterile ultrapure deionized water to reconstitute, the concentration is 50 μg / ml, use the flow detection buffer to prepare a working solution with a concentration of 1 μg / ml, and set aside.
[0075] 3. Add 100 μl of sterile ultrapure deionized water to reconstitute the antibody positive standard and antibody isotype control sample, the concentration is 100 μg / ml, and then dilute a series of concentration gradients with flow detection buffer, the recommended concentration is 0.003 μg / ml ml, 0.03μg / ml, 0.3μg / ml and 3μg / ml, for l...
Embodiment 3
[0083] Embodiment 3, accelerated stability verification
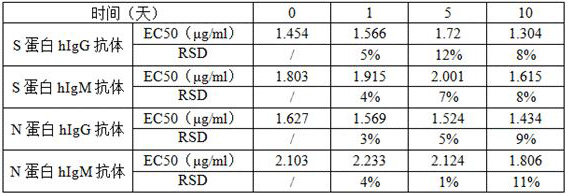
[0084] Store the kit in Example 2 at 37°C for 0, 1, 5, and 10 days, and then detect the positive standard of the new coronavirus antibody. Each sample has 8 concentration gradients. Statistical analysis is performed on the detection data. The acceptance criteria are: compared with the control group (0 hour group), EC 50 The RSD value of the value is ≤20.0%. The measurement results at different times are shown in Table 1. Compared with the control group, the RSDs of EC50 values were all less than 20%. The kits can be stored at -20°C for at least 12 months.
[0085] Table 1 The results of the accelerated stability test of the kit
[0086]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com