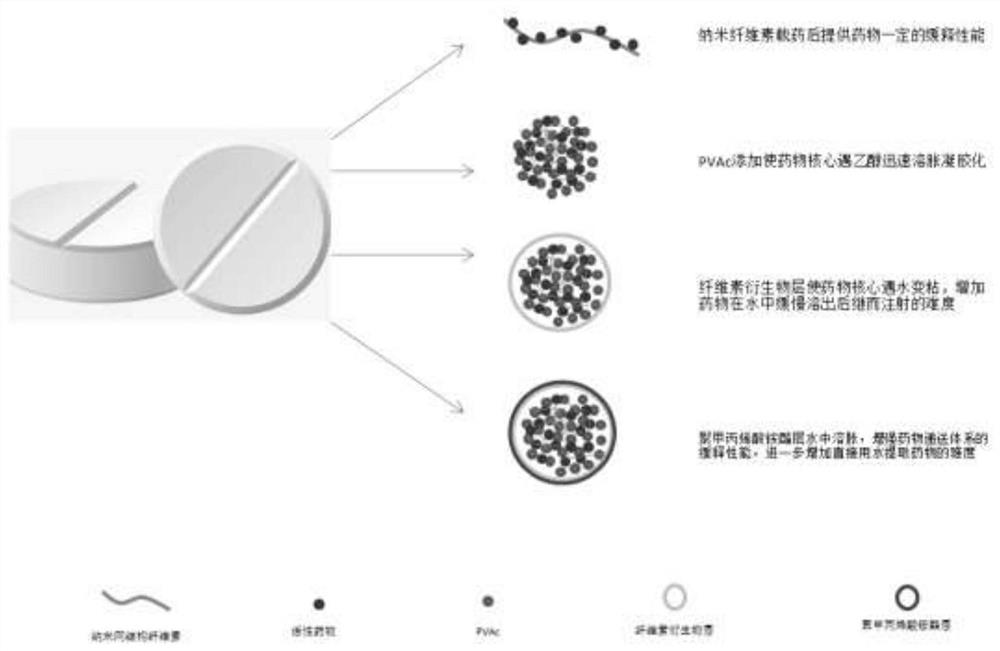
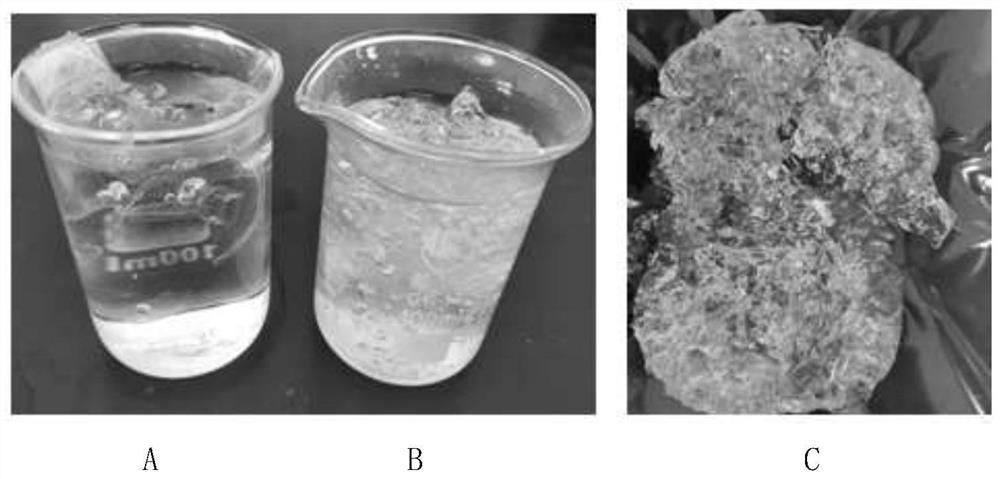
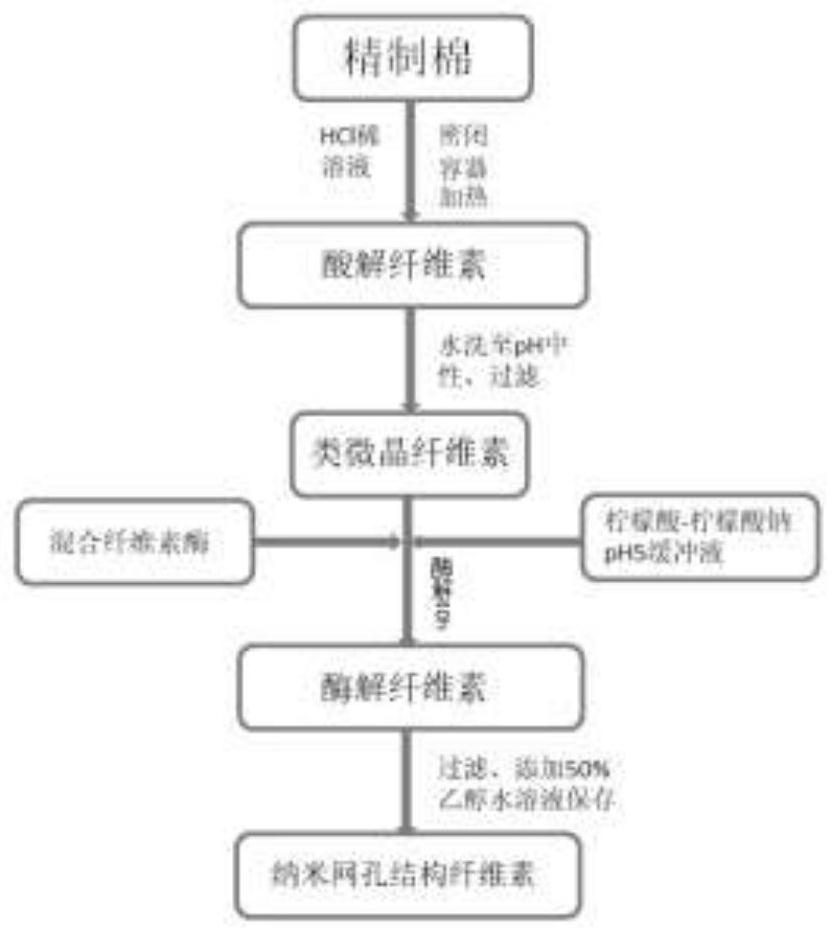
A kind of anti-abuse oral sustained-release opioid tablet and preparation method thereof
A technology for opioid drugs and sustained-release tablets, which is applied in drug combination, drug delivery, and pharmaceutical formulations. It can solve the problems of polymers being easily oxidized, difficult to extract, and high production costs, and achieve obvious sustained-release effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Modified Ammonium Polymethacrylate I
[0072] 1) Preparation method
[0073] The same as "Chinese Pharmacopoeia 2015 Edition" polyammonium methacrylate I, but the ratio of the three monomers is changed from the original: methyl methacrylate: ethyl acrylate: trimethylammonium ethyl methacrylate = 60:30 : 10, changed to methyl methacrylate:ethyl acrylate:trimethylammonium ethyl methacrylate == 55:30:15.
[0074] 2) Main performance
[0075] The main properties of the synthesized polymer are similar to those of polyammonium methacrylate I and are soluble in ethanol, but the modified polyammonium methacrylate I is not only soluble in ethanol, but also swellable and even soluble in water. figure 2 shown.
[0076] Modified polyammonium methacrylate I with a content of 11%-14% trimethylammonium chloride methacrylate can swell in water, and the dissolution rate increases with the increase of ammonium monomer content, and the swelling is slower in cold water. In ho...
Embodiment 2
[0078] The flow chart of the preparation process of mesh nanocellulose is shown in image 3 , the preparation method is detailed in Example 3 of CN110452305A. from Figure 4 It can be seen that the particle size of the self-made cellulose is between 500-5000nm, the average particle size is about 2025nm, and the overall size is still in the micrometer range; it has no potential poisoning and inflammatory risks of nanocellulose (≤100nm) to the human body, and can be directly used as medicine Use with excipients. from Image 6 The atomic force microscope picture shows that some grooves have been carved on the surface of the cotton fiber after gas-solid acid hydrolysis. After enzymatic hydrolysis, the surface roughness is increased, and the grooves are more and deeper. from Figure 7 It can be seen from the scanning electron microscope that the surface of the cotton fiber is roughened after gas-solid acid hydrolysis, and further degraded by cellulase to make the cellulose form...
Embodiment 3
[0080] Since oxycodone hydrochloride is a controlled drug, metformin was used instead of oxycodone hydrochloride in formula screening.
[0081]
[0082]
[0083] Prescription A, B, C preparation method:
[0084] (1) Prepare modified ammonium polymethacrylate I solution (11%) ethanol solution for use.
[0085] (2) Mix the prescribed amount of sodium carboxymethyl cellulose (CMC-Na) with ammonium polymethacrylate I solution (11%) ethanol solution, and dry in an oven at 50° C. for 1 hour.
[0086] (3) Mix the recipe quantities of metformin, vinyl acetate, PVP K30 and the mixture in (3) evenly, and press into tablets.
[0087] Prescription D, E preparation method:
[0088] (1) Add the metformin saturated aqueous solution, the metformin saturated alcohol solution and the cellulosic alcohol solution of the mesh structure of the recipe quantity into the reaction kettle and mix uniformly.
[0089] (2) the mixture in the step (1) is dried by spray drying method, and the spray ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com