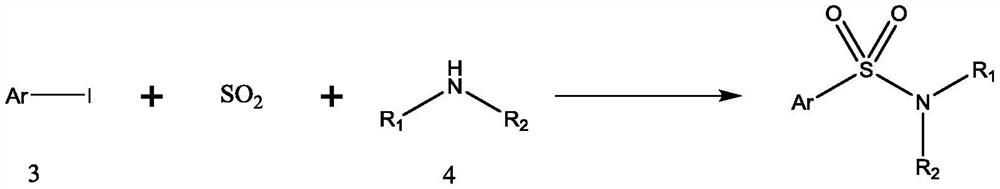
Synthesis method of sulfanilamide compound
A synthetic method and compound technology, applied in the preparation of sulfonamides, organic chemistry, sulfide preparation, etc., can solve the problems of many steps, long reaction routes, and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1. Preparation of compound 5a
[0073]
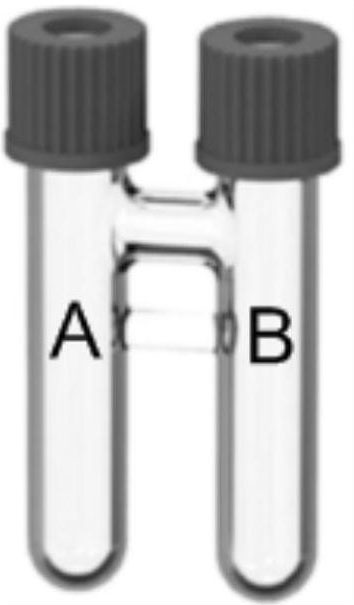
[0074] In a glove box filled with high-purity argon, DMAP (61.0mg, 2.5equiv), nBuPAD 2 (14.4mg, 20.0mol%), Pd(acac) 2 (7.6mg, 12.5mol%), 4-iodo-1,2-dimethoxybenzene 3a (0.2mmol, 1.0equiv), N-methyl-2-phenylethane-1-amine 4a (0.52mmol ,2.6equiv) add in order figure 1 In tube B equipped with a magnetic stirring bar, add 1ml DMSO at the same time. Quickly weigh compound 1 into tube A equipped with a magnetic stirrer, add 1ml of tetradecane, and then add compound 2 into tube A. Quickly tighten the bottle caps of tubes A and B, then take out the glove box, place the above device on a magnetic stirrer at 95°C and stir for 24 hours. Extract with ethyl ester and water (15ml×3 times), combine the ethyl acetate layers, dry over anhydrous sodium sulfate, concentrate, add 200-300 mesh silica gel and mix to dryness, and obtain the compound 5a shown in formula 1 through column chromatography. Yellow liquid, yield 74%.
[00...
Embodiment 2
[0076] Embodiment 2. Preparation of compound 5b
[0077] The preparation method is the same as that of compound 5a, except that 4-iodo-1,2-dimethoxybenzene is replaced by 1-ethoxy-4-iodobenzene to obtain compound 5b, a white solid, with a yield of 63%.
[0078] Compound 5b: 1 H NMR (400MHz, CDCl 3)δ7.70(d, J=8.8Hz, 2H), 7.34-7.23(m, 3H), 7.21(d, J=6.8Hz, 2H), 6.96(d, J=8.8Hz, 2H), 4.10( q,J=6.8Hz,2H),3.28-3.24(m,2H),2.90-2.86(m,2H),2.76(s,3H),1.46(t,J=6.8Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ162.4, 138.5, 129.6, 129.3, 129.0, 128.7, 126.7, 114.7, 64.1, 52.0, 35.3, 35.0, 14.8; HRMS m / z calculated for C 17 h 21 NO 3 S[M+H] + :320.1315,found:320.1316.
Embodiment 3
[0079] Embodiment 3. Preparation of compound 5c
[0080] The preparation method is the same as that of compound 5a, except that 4-iodo-1,2-dimethoxybenzene is replaced with 1-iodo-4-phenoxybenzene to obtain compound 5c, a yellow liquid, with a yield of 62%.
[0081] Compound 5c: 1 H NMR (400MHz, CDCl 3 )δ7.70(d, J=8.8Hz, 2H), 7.43-7.39(m, 2H), 7.32-7.28(m, 2H), 7.23-7.18(m, 4H), 7.07(d, J=7.6Hz ,2H),7.02(d,J=8.8Hz,2H),3.29-3.25(m,2H),2.89-2.86(m,2H),2.77(s,3H); 13 C NMR (100MHz, CDCl 3 )δ161.6, 155.3, 138.4, 131.6, 130.3, 129.6, 128.9, 128.7, 126.7, 125.0, 120.4, 117.7, 51.9, 35.3, 35.0; HRMS m / z calculated for C 21 h 21 NO 3 S[M+Na] + :390.1134,found:390.1136.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com