Moxifloxacin drug derivatives as well as preparation method and application thereof
A technology of moxifloxacin and derivatives, which is applied in the field of biomedical materials, can solve the problems of less application of fluorescent properties and drug activity, and few researches on drug fluorescence properties, and achieve excellent antibacterial effect, good antibacterial activity, and maintain activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of a kind of moxifloxacin drug derivative (MXF-P)
[0061] The synthetic route is as follows:
[0062]
[0063] MXF-P: Dissolve compound 1 (304mg, 0.6mmol) and MXF-HCl (219mg, 0.5mmol) in the mixed solution of MeCN:DMF=9mL:1mL, add KHCO 3 (84mg, 1.0mmol); the reaction mixture was heated to 90°C and reacted for 12 hours. After the solvent was removed by rotary evaporation under reduced pressure, water was added to the mixture. It was then extracted with DCM (3×30ml); the combined organic layers were washed with anhydrous Na 2 SO 4 Drying, concentration, and silica gel column purification (eluent DCM:MeOH=20:1) gave yellow solid MXF-P with a yield of 58%. 1 H NMR (500MHz, CDCl 3 )δ15.16(brs,1H),8.37(s,1H),7.84–7.75(m,9H),7.71–7.65(m,7H),4.02–3.99(m,1H),3.80–3.71(m, 3H),3.65–3.55(m,5H),3.17(brs,1H),2.76(brs,1H),2.53(brs,1H),2.39–2.23(m,3H),1.82–1.41(m,10H) ,1.37–1.17(m,5H),0.99–0.92(m,2H). 13 C NMR (125MHz, CDCl 3 )δ176.7, 167.2, 153.8 (d, J C-F =24...
Embodiment 2
[0065] Preparation of anion in the structure of moxifloxacin derivatives
[0066] will get Br - The compound MXF-P was dissolved (0.2mmol, 166mg) in acetone, and an aqueous solution of potassium hexafluorophosphate (0.3mmol, 55mg) was added and stirred for 2h. The mixture was added water and filtered. The filtered solid is washed with water to remove inorganic salts, and the product containing hexafluorophosphate anion can be obtained after drying.
Embodiment 3
[0068] Characterization of Absorption and Fluorescence Spectra of Moxifloxacin Derivatives
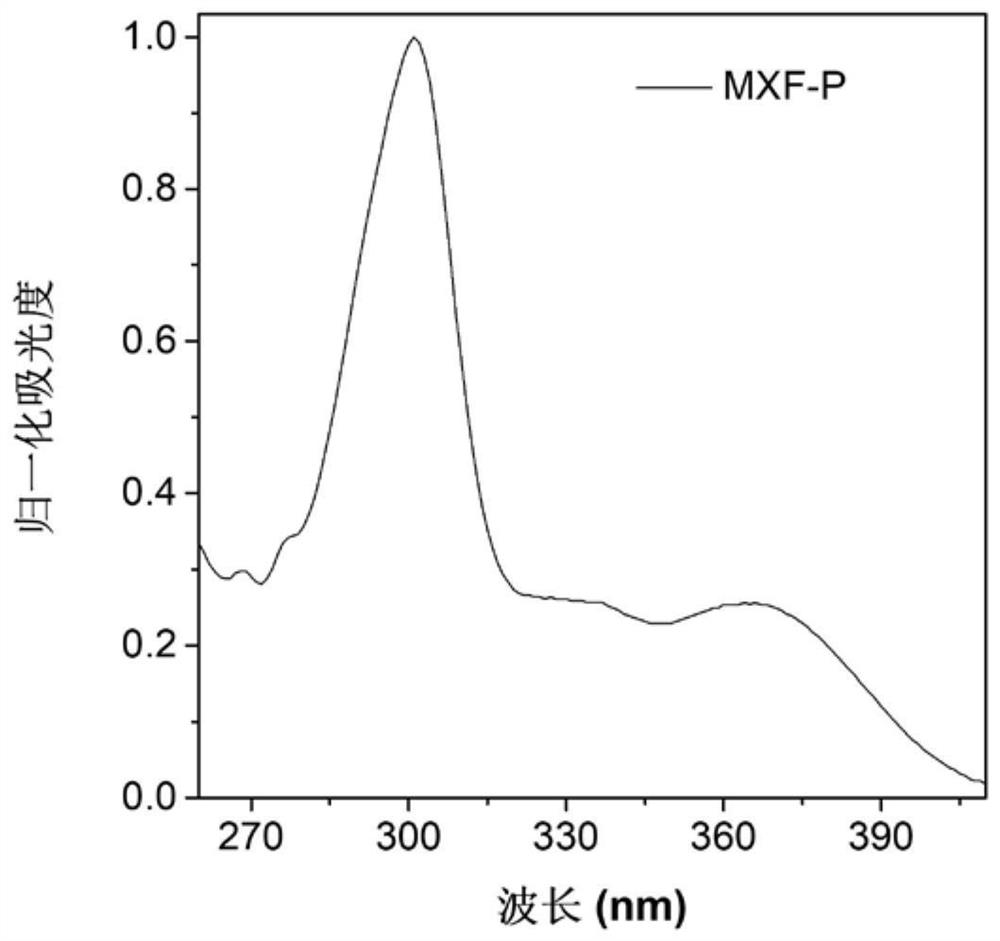
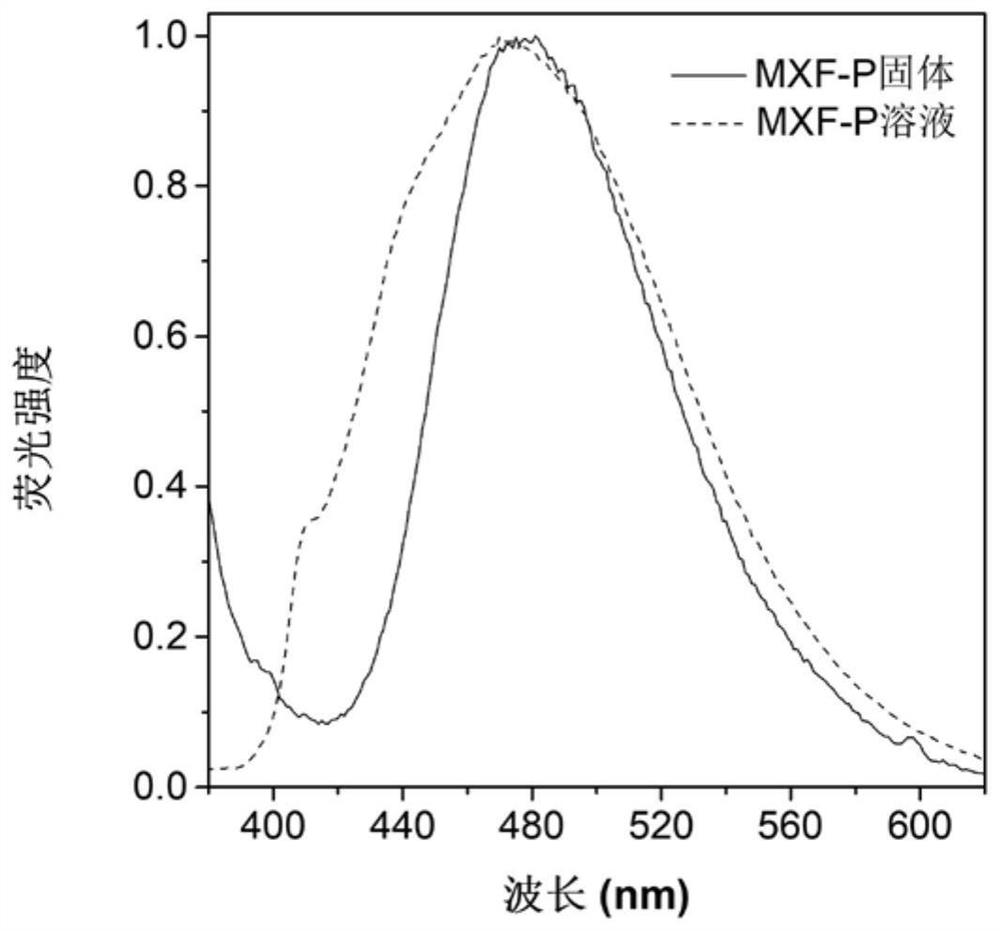
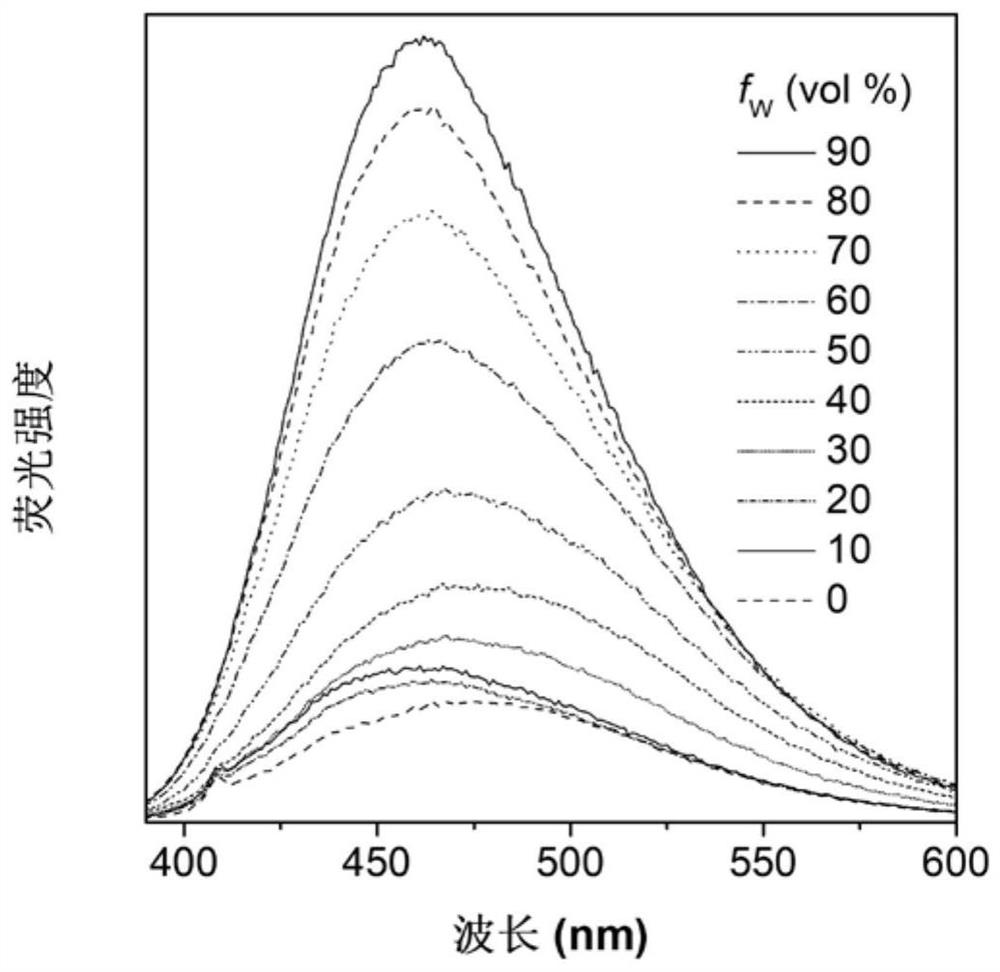
[0069] Figure 1A Based on the absorption spectrum of the material MXF-P obtained in Example 1 in DMSO, it can be seen that the main absorption peak of the molecule is around 365nm; Figure 1B It is the fluorescence emission spectrum of MXF-P in DMSO solvent and solid state, it can be seen that the emission peak of the molecule is mainly around 475nm; Figure 1C for with H 2 O content increased in MXF-P (10 μM) in DMSO / H 2 Fluorescence emission spectrum in O(v / v) mixed solvent; when the molecule is in the solution of DMSO, the luminescence is weak, and it can be found that the fluorescence intensity is continuously enhanced with the addition of water. When the water content reaches 90%, The fluorescence intensity reaches a maximum. This phenomenon is due to the continuous enhancement of fluorescence due to the aggregation of MXF-P molecules as the proportion of poor solvent water in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com