Open-ring cucurbituril column aromatic hydrocarbon double-main-body compound and preparation method and application thereof
A technology of cucurbituril and pillar aromatics, which is applied in the field of double-subject compounds of ring-opening cucurbituril pillar aromatics and its preparation, achieving the effects of good quality, easy manipulation and efficient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
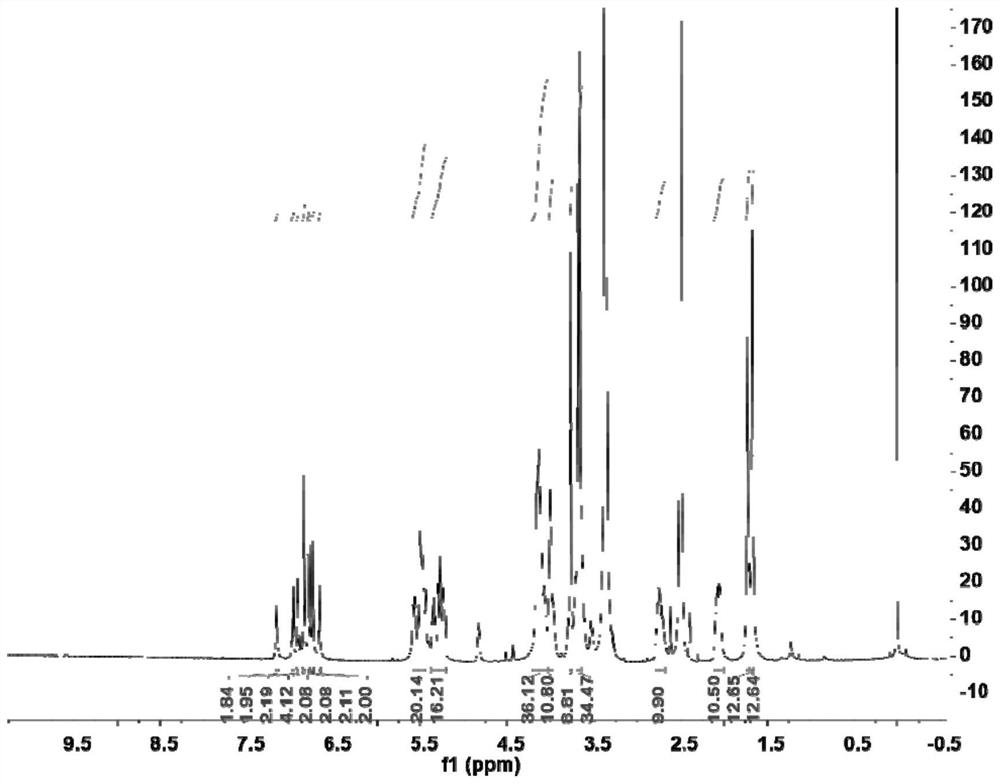
[0033] Example 1: In this example, the ring-opened cucurbituril columnarene double-host compound and its preparation are as follows:
[0034]
[0035] where R is (CH 2 ) m SO 3 Na, where m=3, y=1, k=1, n=1;
[0036]During the preparation, take dicarboxylic acid columnarene (25.14mg, 0.03mmol) and halogen-modified open-ring cucurbituril (164.64mg, 0.12mmol) and dissolve them in 20mL of N,N-dimethylformamide solution, and then react Triethylamine (36.43mg, 0.36mmol) was added to the solution, and reacted at 60°C for 12h; after the reaction was completed and cooled to room temperature, the reaction solution was poured into methanol to produce a precipitate, filtered by suction, and the solid was dissolved in water, and placed in a dialysis bag ( MW=2000) for 48 hours of dialysis, after the dialysis, the dialysate in the dialysis bag was spin-dried, and dried under vacuum at 30°C to obtain the ring-opened cucurbituril columnarene compound, a white solid of 71.17 mg, yield: 7...
Embodiment 2
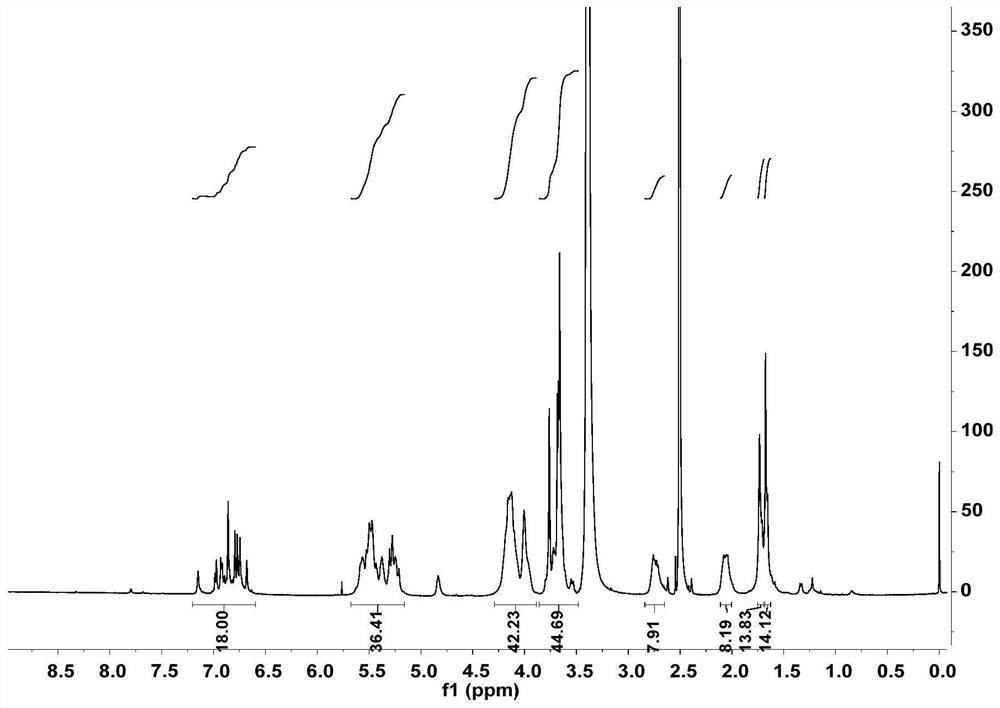
[0038] Example 2: In this example, the ring-opened cucurbituril columnarene double-host compound and its preparation are as follows:
[0039]
[0040] where R is (CH 2 ) m SO 3 Na, where m=3, y=1, k=1, n=2;
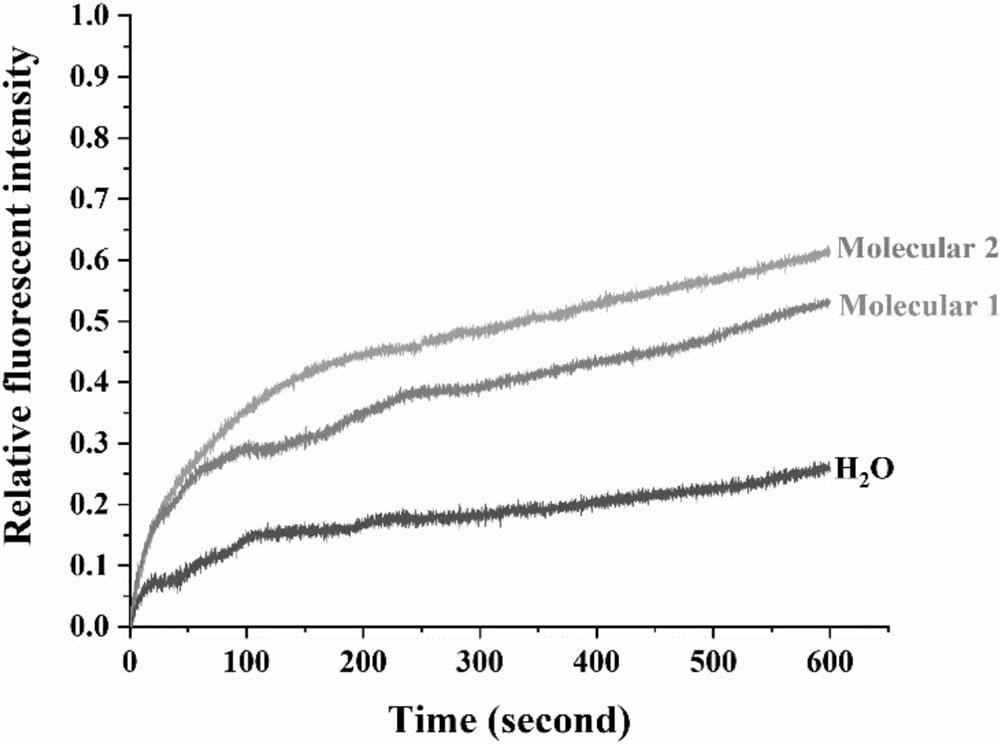
[0041] During the preparation, dicarboxylic acid pyrene (25.14mg, 0.03mmol) and halogen-modified open-ring cucurbituril (84.00mg, 0.06mmol) were dissolved in 20mL of tetrahydrofuran solution, and then sodium carbonate (19.08mg , 0.18mmol), reacted at 70°C for 18h, after the reaction was completed and cooled to room temperature, the reaction solution was poured into acetone to produce a precipitate, suction filtered, and the solid was dissolved in water, and then dialyzed in a dialysis bag (MW=2000) for 72h. After the dialysis is finished, the dialysate in the dialysis bag is spin-dried and dried under vacuum at 45° C. to obtain the split-ring cucurbituril columnarene double-host compound molecule 2 (see Figure II ), white solid 68.41 mg, yield: 66.08%.
Embodiment 3
[0042] Example 3: In this example, the ring-opened cucurbituril columnarene double-host compound and its preparation are as follows:
[0043]
[0044] where R is -(CH 2 ) x -OPO 3 K 2 , where x=2, y=1, k=1, n=1;
[0045] During the preparation, dicarboxylic acid cylarene (25.14mg, 0.03mmol) and open-ring cucurbituril (133.57mg, 0.09mmol) were dissolved in 20mL of dimethyl sulfoxide solution, and potassium carbonate (62.19 mg, 0.45mmol), reacted at 100°C for 36h; after the reaction was completed and cooled to room temperature, the reaction solution was poured into diethyl ether to produce a precipitate, filtered with suction, and the solid was dissolved in water and dialyzed in a dialysis bag (MW=2000) for 48h, and dialyzed After the end, the dialysate in the dialysis bag was spin-dried and dried under vacuum at 60° C. to obtain the ring-opened cucurbituril columnarene dual-host compound, 76.86 mg of white solid, yield: 70.26%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com