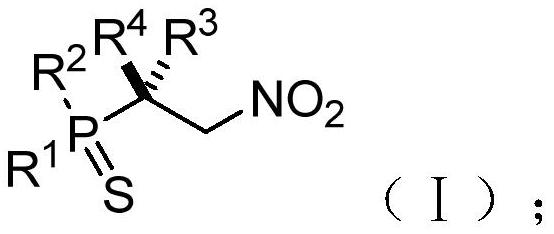
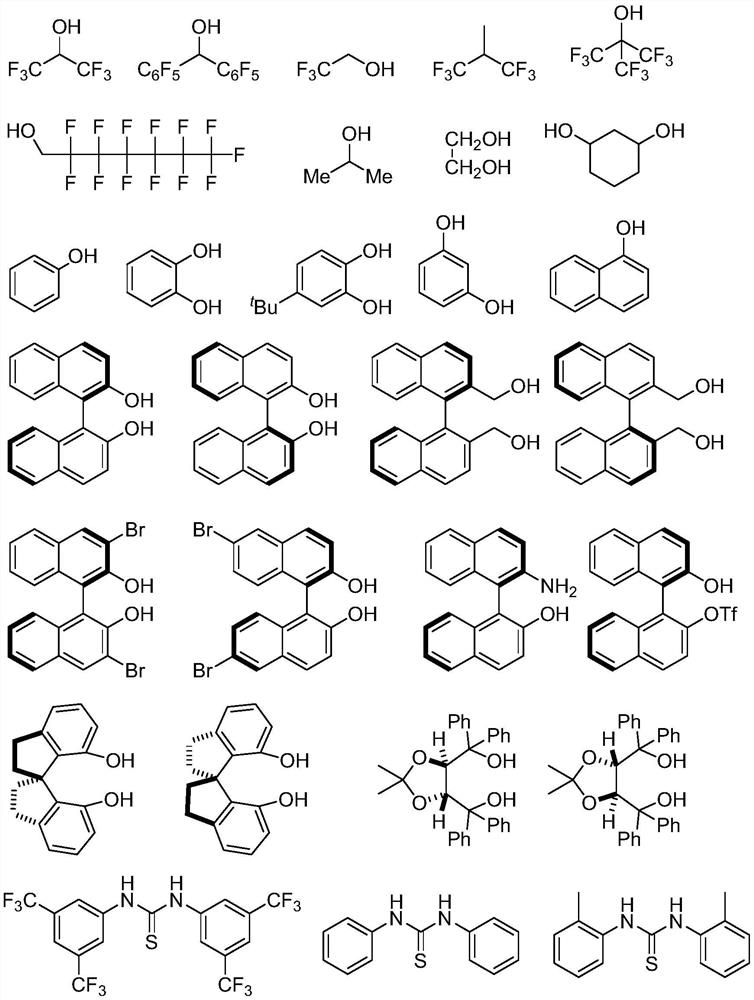
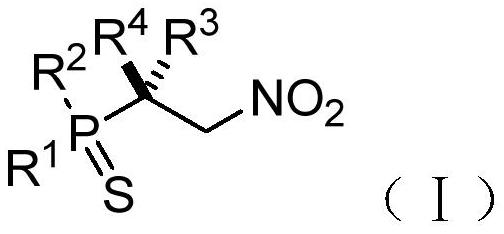
Chiral phosphorus-sulfur compound and Michael addition method thereof
A technology for phosphorus-sulfur compounds and compounds, applied in the field of chiral phosphorus-sulfur compounds and their Michael addition, can solve the problems of single activation mode and limited types of nucleophiles for phosphorus-sulfur compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] This example provides a (S)-diphenyl(1,1,1-trifluoro-3-nitro-2-phenylpropan-2-yl)phosphorus-sulfur compound and a preparation method thereof. The structural formula of the (S)-diphenyl (1,1,1-trifluoro-3-nitro-2-phenylpropan-2-yl) phosphorus sulfur compound is shown in the molecular structural formula I1 below:
[0150]
[0151] Its preparation steps are as follows:
[0152]In a 10ml test tube, the indenol-derived triazole carbene catalyst (0.01mmol, 0.1 equivalent (equiv.)) and the phosphorus-sulfur nucleophile (0.1mmol, 1.0equiv.), binaphthol (R- BINOL, 0.02mmol, 0.2equiv.) was dissolved in 1.6mL of a pretreated mixed solvent of ethylbenzene and cyclohexane with a ratio of 9:1, sealed with a rubber stopper, and then replaced with gas under an argon atmosphere (3 times) , slowly added lithium bis(trimethylsilyl)amide (LiHMDS) (1 mol / L, tetrahydrofuran / ethylbenzene solution, 8 μL, 0.08 equiv.), and replaced the gas again under an argon atmosphere (3 times). The tub...
Embodiment 2
[0155] This example provides a (S)-di-p-tolyl (1,1,1-trifluoro-3-nitro-2-phenylpropan-2-yl) phosphorus sulfur compound and a preparation method thereof. The structural formula of the (S)-di-p-tolyl (1,1,1-trifluoro-3-nitro-2-phenylpropan-2-yl) phosphorus sulfur compound is shown in molecular structural formula I2 below:
[0156]
[0157] Its preparation method refers to the preparation method of (S)-diphenyl (1,1,1-trifluoro-3-nitro-2-phenylpropan-2-yl) phosphorus sulfur compound in Example 1, the difference is that The p-tolylphosphosulfur compound (0.1 mmol) was used instead of the diphenylphosphosulfur compound. The reaction solution was directly separated and purified by silica gel column chromatography (ethyl acetate and n-hexane as eluents) to obtain the target product, a white solid, with a yield of 94% and an ee value of 95%.
[0158] The prepared product I2 is subjected to characterization data analysis, and the result is 1 H NMR (400MHz, CDCl 3 )δ7.91(dd, J=12....
Embodiment 3
[0160] This example provides a (S)-di-p-tolyl(1,1,1-trifluoro-2-(4-methoxyphenyl)-3-nitropropan-2-yl)phosphorus Compounds and methods for their preparation. (S)-two-p-tolyl (1,1,1-trifluoro-2-(4-methoxyphenyl)-3-nitropropane-2-yl)phosphorus sulfur compound has the following molecular structure formula I3 Shown:
[0161]
[0162] Its preparation method refers to the preparation method of (S)-diphenyl (1,1,1-trifluoro-3-nitro-2-phenylpropan-2-yl) phosphorus sulfur compound in Example 1, the difference is that Use p-tolyl phosphosulfur compound (0.1mmol) instead of diphenylphosphorsulfur compound, (E)-1-methoxy-4-(3,3,3-trifluoro-1-nitropropane-1 -en-2-yl)benzene was substituted for (E)-(3,3,3-trifluoro-1-nitroprop-1-en-2-yl)benzene (0.12 mmol). The reaction solution was directly separated and purified by silica gel column chromatography (ethyl acetate and n-hexane as eluents) to obtain the target product, a white solid, with a yield of 98% and an ee value of 93%.
[0163]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com