Streptococcus suis vaccine recombinant protein GSE and preparation method and application thereof
A recombinant protein, Streptococcus suis technology, which is applied in the biological field to achieve the effect of good practicability and efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The preparation method of the above-mentioned recombinant protein comprises the following steps:
[0056] (1) B cell and helper T cell epitope screening;
[0057] (2) B cell and helper T cell epitope tandem gene design and plasmid construction;
[0058] (3) Prokaryotic expression and purification of the recombinant protein to obtain the recombinant protein GSE of the Streptococcus suis vaccine.
[0059] Preferably, the specific method of step (1) is: according to the amino acid sequence of Streptococcus suis glyceraldehyde triphosphate dehydrogenase (GAPDH), enolase (Enolase), DNA nuclease (SsnA), apply bioinformatics software , analyze the primary structure of the protein, exclude the transmembrane region and the signal peptide region during epitope screening, then predict the B cell and helper T cell epitope, and finally analyze the secondary structure of the protein to further verify the screened epitope Whether it is in the area of antigenicity and hydrophilicit...
Embodiment 1
[0076] Antigen epitope screening:
[0077] 1. Protein primary structure analysis
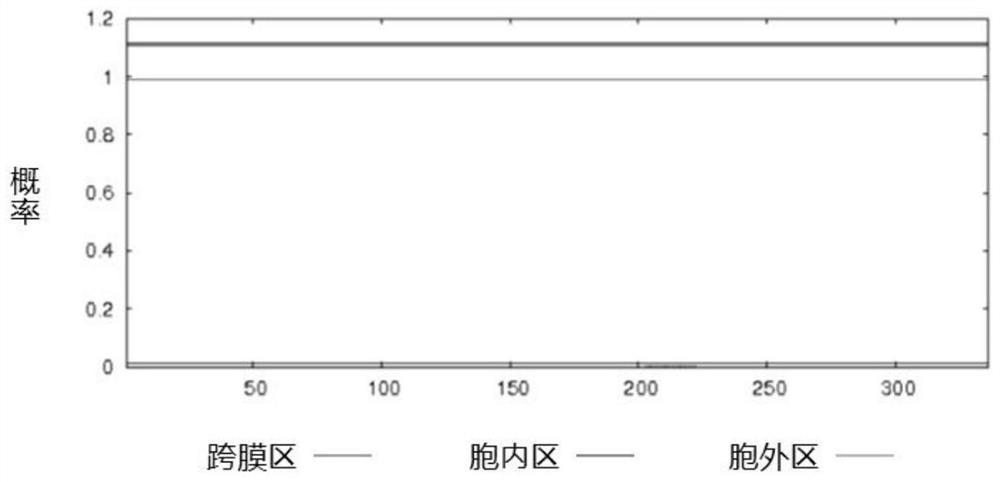
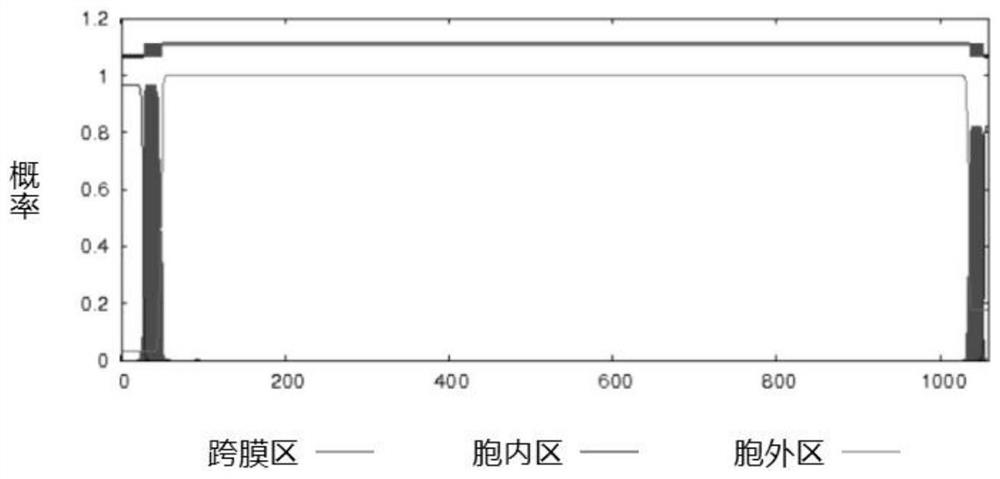
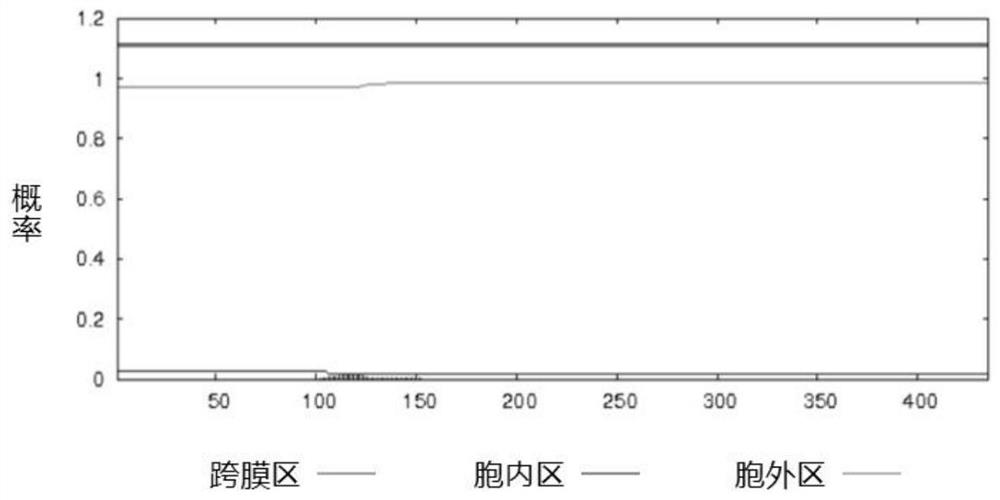
[0078] The protein amino acid sequences of Streptococcus suis GAPDH, SsnA, and Enolase published by NCBI are respectively AKG39592.1, ABP90934.1, and ACS66679.1, and the sequence lengths are 336aa, 1059aa, and 435aa, respectively. According to the protein amino acid sequences of GAPDH, SsnA and enolase, the transmembrane region of the protein was analyzed by TMHMM tool. The SignalP tool was used to analyze the protein signal peptide region.
[0079] The results of analysis using the TMHMM tool showed that there was no transmembrane region in the GAPDH protein, and its 336 amino acids were all extracellular fragments (such as figure 1 shown). The SsnA protein has two transmembrane regions, the N-terminal amino acid 1-27 is an intracellular fragment, the 28-50 amino acid is a transmembrane fragment, the C-terminal amino acid 1054-1059 is an intracellular fragment, and the 1036-1053 amino acid i...
Embodiment 2
[0096] Recombinant protein design and plasmid construction:
[0097] The predicted B cell and helper T cell epitopes were spliced sequentially in the order of GAPDH-SsnA-Enolase, and a dendritic cell targeting peptide sequence (sequence FYPSYHSTPQRP) was added to the N-terminus of the recombinant polypeptide. Each peptide segment Four glycine (GGGG) short peptides were used to connect them. After the concatenation is completed, the amino acid sequence of the recombinant protein is obtained, with a total of 373 amino acids, as shown in SEQ ID NO.1.
[0098] The amino acid sequence is translated into a nucleotide sequence, and then the obtained sequence is optimized according to the codon preference of E. coli, and a restriction site BamH1 is added to the N-terminal of the sequence, and a restriction site XhoI is added to the C-terminal of the sequence to finally obtain the recombinant The nucleotide sequence of the protein GSE has a total of 1131 nucleotides, as shown in SEQ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com