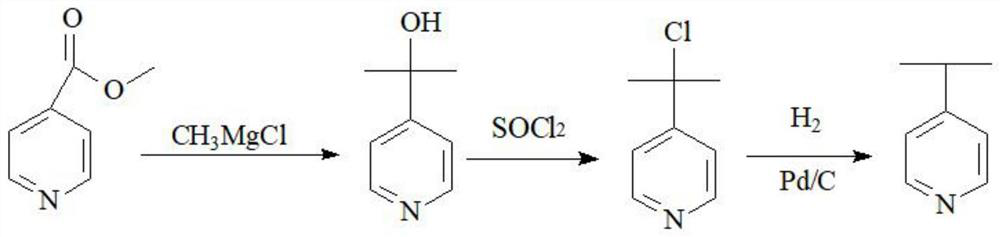
Novel method for preparing 4-isopropylpyridine
A technology of propylpyridine and isopropanolpyridine, which is applied in the direction of organic chemistry, can solve the problems of cooling water supply cycle, such as adverse effects, death, a large amount of manpower and working time, and achieve obvious market competitive advantages, simple process operation, Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of 4-isopropanolpyridine:
[0025] The methyl chloride of 8.7kg is passed into the THF of 30kg below 0 DEG C to prepare methyl chloride / THF mixed solution, 3.6kg of dry magnesium is added in the 50L reactor, and the temperature control 5-15 DEG C is added dropwise to the mixed solution of methyl chloride / THF , the dropwise addition was completed, the temperature was kept at 10-15°C and reacted for 1 hour, then slowly warming up to 30°C for 2 hours, adding 30kg tetrahydrofuran and 6.85kg methyl isonicotinate in another 100-liter reactor, and dropping below 30°C of temperature control. Add the prepared methyl chloride Grignard reagent, keep the temperature at 25-30 °C for 12 hours after the dropwise addition, recover the THF to 90 °C under normal pressure, basically no more, lower the temperature below 30 °C, add hydrochloric acid aqueous solution to the system pH to 6-7, Centrifuge to obtain 4-isopropanol pyridine, and oven dry to obtain 5.4 kg of 4-isopropan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com