Pharmaceutical preparation for treating lung diseases, and preparation method thereof
A technology for lung diseases and pharmaceutical preparations, applied in the direction of drug combinations, pharmaceutical formulas, respiratory diseases, etc., to achieve the effect of controllable preparation process and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059]
[0060]
[0061] Preparation Process:
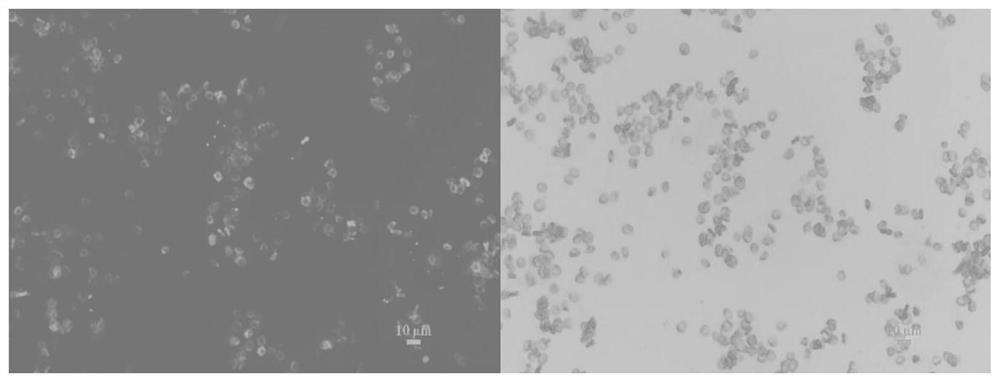
[0062] Weigh 10 mg of bovine serum albumin, dissolve in 5 mL of deionized water; weigh 1.5 mg of FITC, and dissolve in 4 mL of ethanol. The two were added to a round bottom flask and mixed, and reacted in an ice-water bath at 4°C for 8h. The reaction solution was divided into dialysis bags with a molecular weight cut-off of 3500 Da, and dialyzed in deionized water environment to remove free FITC. The fluid in the dialysis bag was collected to obtain FITC-labeled bovine serum albumin for later use. The isolated and purified red blood cells were diluted to 20% with PBS buffer. Weigh 40 mg of tannic acid and dissolve it in 1 mL of deionized water to prepare a tannic acid mother liquor. Accurately weigh 8.9 mg of lanthanum trichloride heptahydrate, and dissolve it in 1 mL of deionized water to prepare a mother liquor of lanthanum trichloride. Add 100 μL of red blood cells to 400 μL of PBS buffer, and mix by inverting up and...
Embodiment 2
[0065]
[0066]
[0067] Preparation Process:
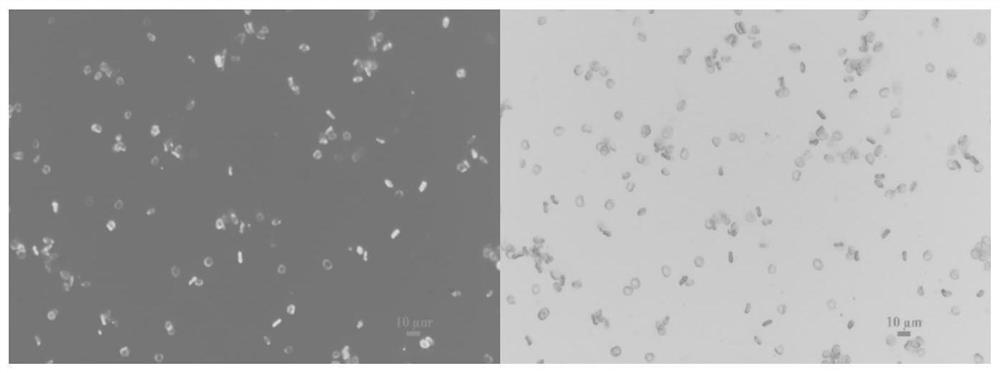
[0068] Weigh 10 mg of bovine serum albumin, dissolve in 5 mL of deionized water; weigh 1.5 mg of FITC, and dissolve in 4 mL of ethanol. The two were added into a round-bottomed flask and mixed, and reacted for 8 hours at 4°C in an ice-water bath. The reaction solution was divided into dialysis bags with a molecular weight cut-off of 3500 Da, and dialyzed in deionized water environment to remove free FITC. The fluid in the dialysis bag was collected to obtain FITC-labeled bovine serum albumin for later use. The isolated and purified red blood cells were diluted to 20% with PBS. Weigh 40 mg of tannic acid and dissolve it in 1 mL of deionized water to prepare a tannic acid mother liquor. Weigh 3.9 mg of ferric chloride hexahydrate and dissolve it in 1 mL of deionized water to prepare ferric chloride hexahydrate mother liquor. Add 100 μL of red blood cells to 400 μL of PBS and mix by inverting up and down. Add 5 μL of tann...
Embodiment 3
[0071]
[0072]
[0073] Preparation Process:
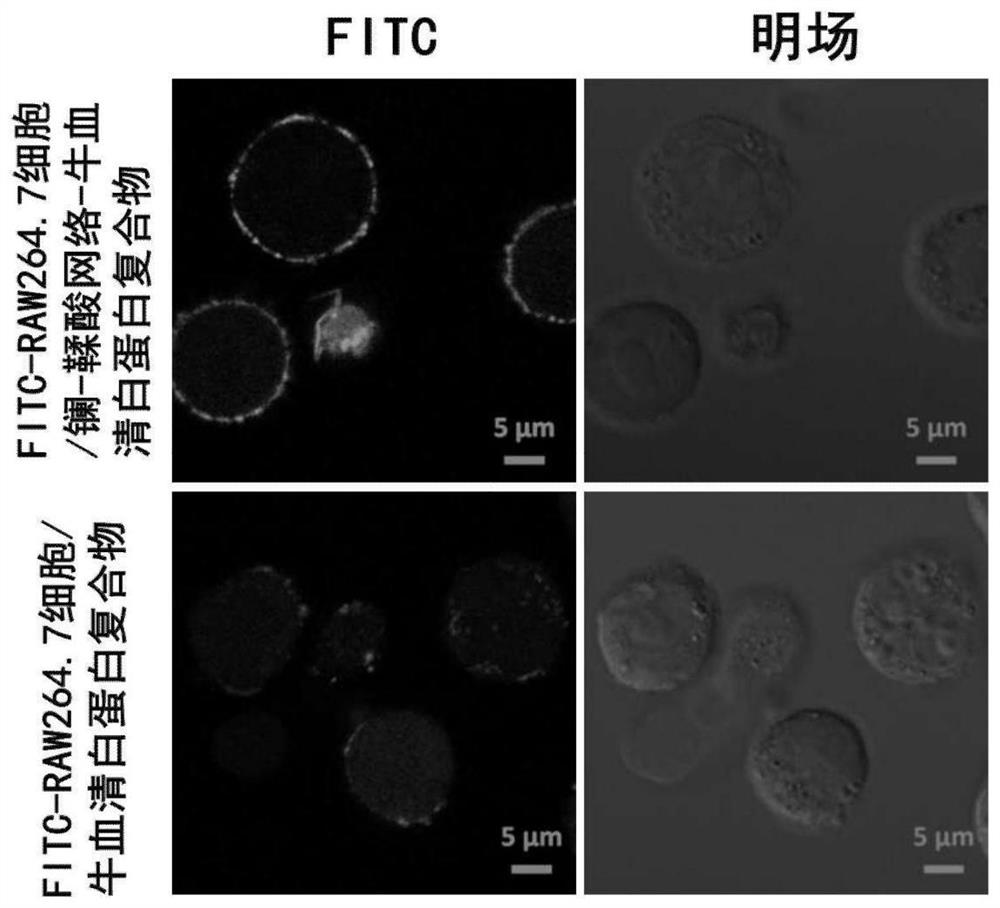
[0074] Weigh 10 mg of bovine serum albumin, dissolve in 5 mL of deionized water; weigh 1.5 mg of FITC, and dissolve in 4 mL of ethanol. The two were added into a round-bottomed flask and mixed, and reacted for 8 hours at 4°C in an ice-water bath. The reaction solution was divided into dialysis bags with a molecular weight cut-off of 3500 Da, and dialyzed in deionized water environment to remove free FITC. The liquid in the dialysis bag was collected to obtain FITC-bovine serum albumin for later use. Dilute RAW264.7 cells with PBS to 2 × 10 6 ~4×10 6 individual / mL. Weigh 40 mg of tannic acid and dissolve it in 1 mL of deionized water to prepare a tannic acid mother liquor. Accurately weigh 8.9 mg of lanthanum trichloride heptahydrate, and dissolve it in 1 mL of deionized water to prepare a mother liquor of lanthanum trichloride. Add 100 μL of RAW264.7 cells to 400 μL of PBS, and mix by inverting up and down. Add 5 μL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com