Nickel-cobalt-tungsten polysulfide bifunctional catalyst with core-shell spherical structure as well as preparation method and application of nickel-cobalt-tungsten polysulfide bifunctional catalyst
A catalyst and sulfide technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of slow electron transfer rate, single-component material catalytic activity tends to bottleneck, etc., to achieve preparation Short process, excellent catalytic activity and hydrogen production performance, and high photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
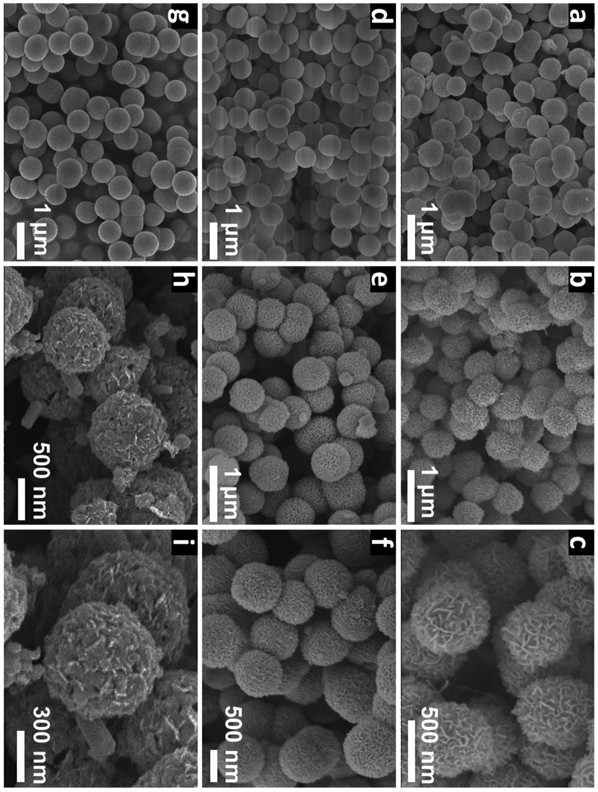
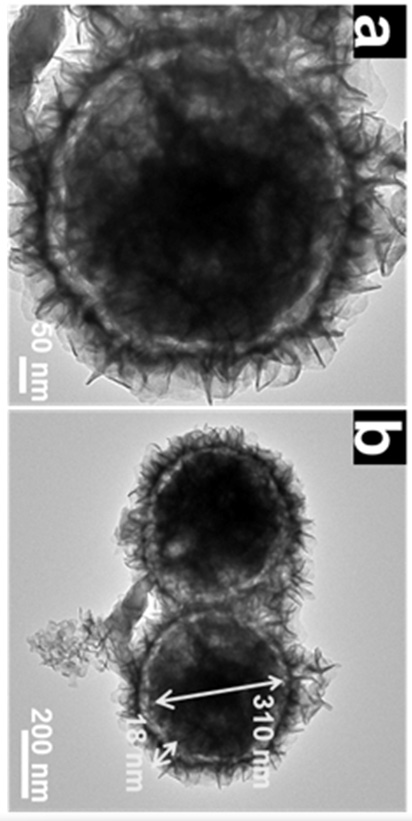
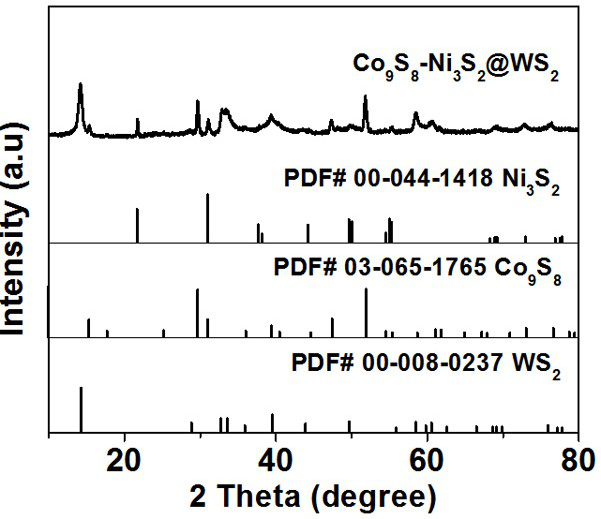
[0033] Disperse 50 mg of cobalt nitrate hexahydrate, 60 mg of nickel nitrate hexahydrate, and 12 mL of glycerin in 50 mL of isopropanol. After magnetic stirring at room temperature for 20 min, pour the mixture into a hydrothermal kettle. Under reaction 8 h. The final nickel-cobalt-glycerol precursor was collected by centrifugation, washed with ethanol several times, and then dried overnight in a vacuum oven at 60 °C. 80 mg nickel-cobalt-glycerol precursor, 75 mg ammonium tungstate and 375 mg thioacetamide were dissolved in 100 mL ethanol, and magnetically stirred at room temperature for 20 min. Then transferred to a hydrothermal kettle for hydrothermal reaction at 160 °C for 8 h. After centrifugal washing and drying, the product was placed in the center of a porcelain boat, heated to 500 °C in a tube furnace, kept for 1 h, and the heating rate was 1 °C min −1 , and finally get the core-shell spherical structure of Co 9 S 8 -Ni 3 S 2 @WS 2 catalyst.
Embodiment 2
[0035] Disperse 50 mg cobalt nitrate hexahydrate, 50 mg nickel nitrate hexahydrate, 20 mL glycerin in 50 mL isopropanol, stir magnetically at room temperature for 20 min, pour the mixture into a hydrothermal kettle, and heat at 180 °C Under reaction for 10 h. The final nickel-cobalt-glycerol precursor was collected by centrifugation, washed with ethanol several times, and then dried overnight in a vacuum oven at 60 °C. 75 mg nickel-cobalt-glycerol precursor, 75 mg ammonium tungstate and 375 mg thioacetamide were dissolved in 75 mL ethanol, and magnetically stirred at room temperature for 20 min. Then transferred to a hydrothermal kettle for hydrothermal reaction at 180 °C for 12 h. After centrifugal washing and drying, the product was placed in the center of a porcelain boat and heated to 600 °C in a tube furnace for 2 h at a heating rate of 2 °C min −1 , and finally get the core-shell spherical structure of Co 9 S 8 -Ni 3 S 2 @WS 2 catalyst.
Embodiment 3
[0037] Disperse 50 mg cobalt nitrate hexahydrate, 80 mg nickel nitrate hexahydrate, and 15 mL glycerin in 70 mL isopropanol, stir magnetically at room temperature for 20 min, pour the mixture into a hydrothermal kettle, and heat at 170 °C Under reaction for 12 h. The final nickel-cobalt-glycerol precursor was collected by centrifugation, washed with ethanol several times, and then dried overnight in a vacuum oven at 60 °C. 85 mg nickel-cobalt-glycerol precursor, 75 mg ammonium tungstate and 375 mg thioacetamide were dissolved in 100 mL ethanol, and magnetically stirred at room temperature for 20 min. Then transferred to a hydrothermal kettle for hydrothermal reaction at 180 °C for 12 h. After centrifugal washing and drying, the product was placed in the center of the porcelain boat and heated to 700 °C in a tube furnace for 2 h at a heating rate of 2 °C min −1 , and finally get the core-shell spherical structure of Co 9 S 8 -Ni 3 S 2 @WS 2 catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com