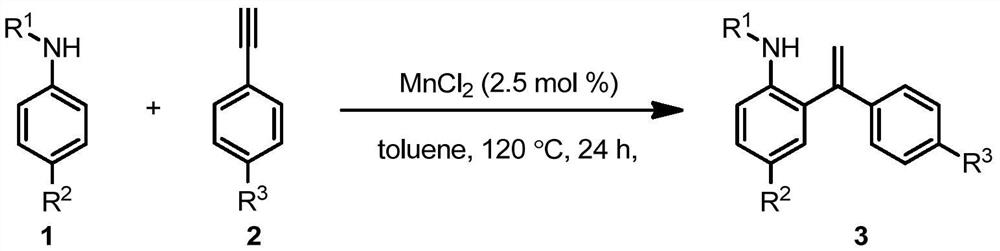
Bivalent manganese catalyzed arylamine ortho-enylation reaction method
A technology of divalent manganese catalysis and divalent manganese, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of large amount of Lewis acid, increased production cost, large amount of catalyst, etc., and achieves good results. Catalytic effect, improved yield, low environmental pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of N-benzyl-2-(1-phenylvinyl)aniline:
[0037]
[0038] In the reaction flask, add 0.20 mmol of 1a and 0.04 mmol of MnCl in sequence 2 , 0.4mmol of 2a and 2.0mL of toluene, stirred at 120°C for 24 hours, cooled to room temperature, added 5mL of ethyl acetate to dilute, washed with 5mL of saturated brine, dried the organic phase with anhydrous magnesium sulfate, and spin-dried the column Chromatography, the eluent was ethyl acetate: n-hexane = 1:100, the product 3a was obtained as 46 mg of white solid, and the yield was 80%. 1 H NMR (400MHz, CDCl 3 ):δ7.39–7.10(m,10H),7.05–6.99(m,2H),6.73(t,J=7.4Hz,1H),6.61(d,J=8.0Hz,1H),5.79(d, J=1.2Hz, 1H), 5.37(d, J=1.2Hz, 1H), 4.21(s, 2H), 4.06 (s, 1H); 13 C NMR (100MHz, CDCl 3 ): δ147.3, 145.2, 139.9, 139.4, 130.7, 129.1, 128.7, 128.6, 128.2, 127.4, 127.1, 127.1, 126.8, 117.0, 116.6, 110.8, 48.0.
Embodiment 2
[0040] Synthesis of N-benzyl-2-(1-(2-chlorophenyl)vinyl)aniline:
[0041]
[0042] In the reaction flask, add 0.20 mmol of 1a and 0.04 mmol of MnCl in sequence 2 , 0.4mmol of 2b and 2.0mL of toluene, stirred at 120°C for 24 hours, cooled to room temperature, added 5mL of ethyl acetate to dilute, washed with 5mL of saturated brine, dried the organic phase with anhydrous magnesium sulfate, and spin-dried the column Chromatography, the eluent was ethyl acetate: n-hexane = 1:100, the product 3b was obtained as a white solid 55mg, and the yield was 86%. 1 H NMR (400MHz, CDCl 3 ):δ7.38–7.11(m,10H),7.00(dd,J=7.6,1.6Hz,1H),6.68–6.66(m,2H),5.73(d,J=1.6Hz,1H),5.60( d,J=1.6Hz,1H),4.56(s,1H),4.29–4.28(m,2H); 13 C NMR (100MHz, CDCl 3 ): δ145.1, 140.9, 139.3, 132.6, 130.8, 130.1, 129.7, 128.8, 128.7, 128.5, 127.2, 127.0, 126.7, 121.0, 116.7, 110.9, 48.2; HRMS(ESI) m / z Calcd for C 2 1H 18 ClN[M+H] + 319.1128, Found 319.1131.
Embodiment 3
[0044] Synthesis of N-benzyl-2-(1-(4-chlorophenyl)vinyl)aniline:
[0045]
[0046] In the reaction flask, add 0.20mmol of 1a and 0.04mmol of MnBr successively. 2 , 0.4mmol of 2c and 2.0mL of toluene, stirred at 120°C for 24 hours, cooled to room temperature, added 5mL of ethyl acetate to dilute, washed with 5mL of saturated brine, dried the organic phase with anhydrous magnesium sulfate, and spin-dried the column layer Analysis, the eluent was ethyl acetate:n-hexane=1:100, the product 3c was obtained as 58 mg of white solid, and the yield was 90%. 1 H NMR (400MHz, CDCl 3 ):δ7.29–7.19(m,8H), 7.10(dd,J=7.2,1.6Hz,1H),7.07–7.03(m,2H),6.74(td,J=7.2,0.8Hz, 1H), 6.63(d, J=8.0Hz, 1H), 5.78(d, J=1.2Hz, 1H), 5.39(d, J=1.2Hz, 1H), 4.23(s, 2H), 3.99(s, 1H); 13 C NMR (100MHz, CDCl 3 ): δ146.0, 145.1, 139.2, 138.2, 134.0, 130.5, 129.2, 128.7, 128.5, 128.0, 127.1, 127.1, 126.7, 117.0, 116.9, 110.8, 48.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com