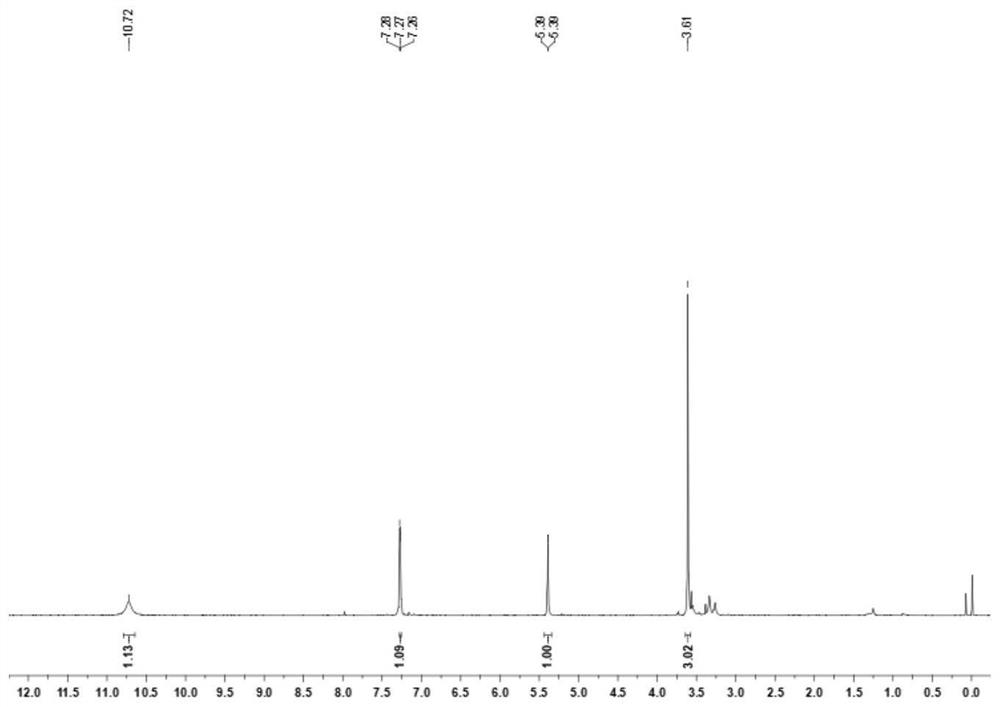
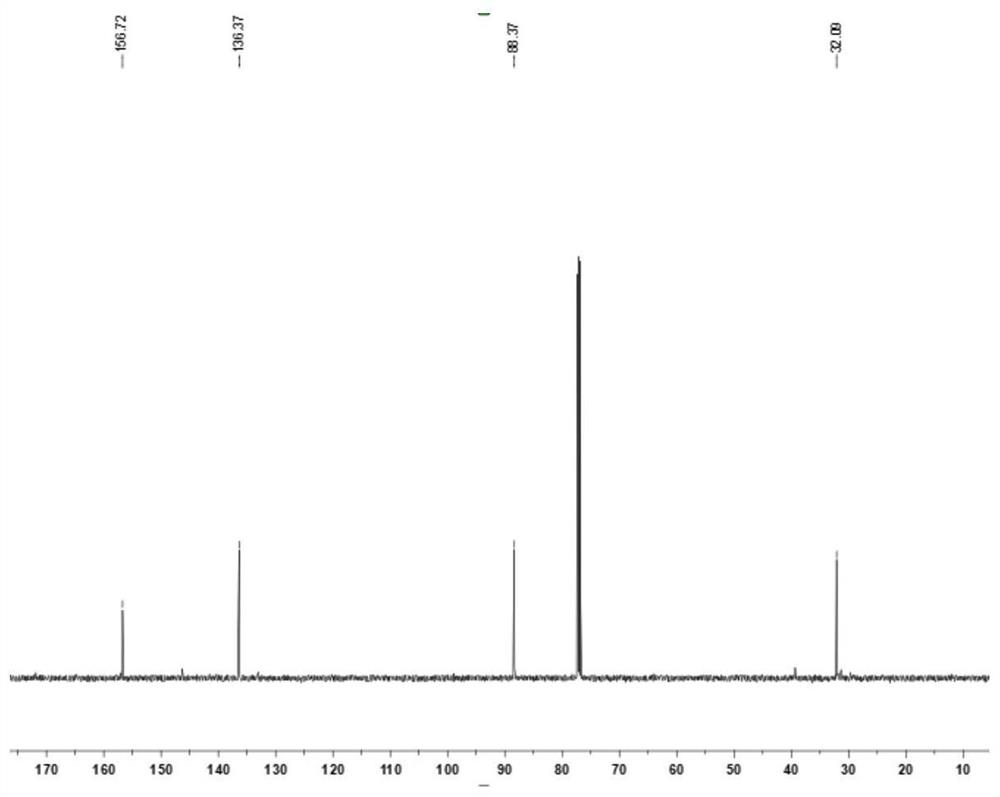
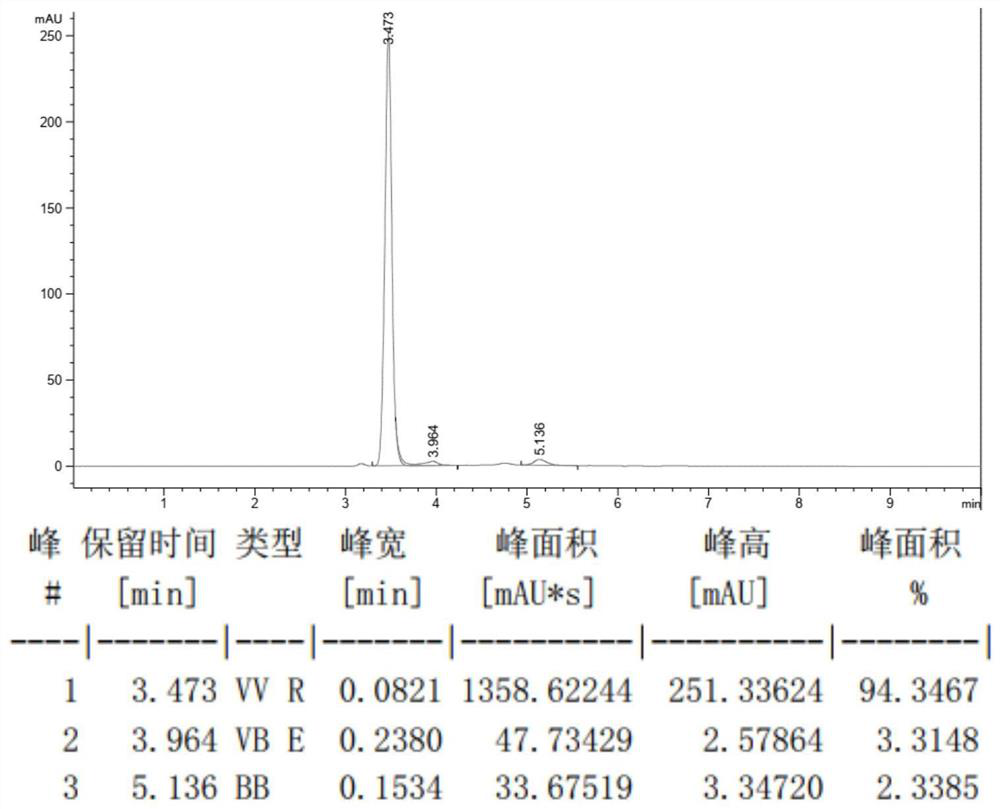
Synthesis method of 1-methyl-5-hydroxy pyrazole
A technology of hydroxypyrazole and synthetic method, applied in the direction of organic chemistry, etc., can solve the problems of easy moisture absorption, low purity, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] At room temperature, add 6.49g (30mmol) of diethyl ethoxymethylenemalonate to a 100mL round bottom flask, weigh 2.34g (33mmol) of tetrahydropyrrole and slowly drop it into the flask, then react at 25°C 3 hours, take by weighing methylhydrazine aqueous solution (40%) 4.49g (methylhydrazine 39mmol) slowly dropwise in the above-mentioned solution, reaction temperature is controlled at 25 ℃, reaction time is 5 hours, then slowly add dropwise 15mL concentrated hydrochloric acid (mass Fraction 37%), a yellow solid precipitated out, then dissolved, and the solution turned into a yellow clear solution. The round bottom flask was then heated to reflux in an oil bath at 100°C for 6 hours. After cooling to room temperature, use 30% mass fraction sodium hydroxide solution to adjust the pH of the reaction solution to be neutral, add NaCl solid until the solution is saturated, use n-butanol to extract the aqueous solution, separate the layers, take the organic layer, and remove most ...
Embodiment 2
[0034]At room temperature, add 10.81g (50mmol) of diethyl ethoxymethylenemalonate to a 100mL round bottom flask, weigh 4.27g (60mmol) of tetrahydropyrrole and slowly drop it into the flask, then react at 30°C For 3 hours, weigh 7.49 g of methylhydrazine aqueous solution (40%) (65 mmol of methylhydrazine) and slowly add it dropwise to the above solution. During the process, a yellow solid precipitated out, then dissolved, and the solution turned into a yellow clear solution. The round bottom flask was then heated to reflux in an oil bath at 110°C for 6 hours. After being cooled to room temperature, adjust the pH of the reaction solution to be neutral with 30% mass fraction sodium hydroxide solution, add Na 2 SO 4 Solid until the solution is saturated, extract the aqueous solution with butyl acetate, separate the layers, take the organic layer, and distill off most of the butyl acetate under reduced pressure, then filter the precipitated residual Na 2 SO 4 , the filtrate was...
Embodiment 3
[0036] At room temperature, add 11.89g (55mmol) of diethyl ethoxymethylenemalonate to a 100mL round bottom flask, weigh 5.12g (72mmol) of tetrahydropyrrole and slowly drop it into the flask, then react at 35°C After 2 hours, weigh 8.24 g of methylhydrazine aqueous solution (40%) (72 mmol of methylhydrazine) and slowly add it dropwise to the above solution. The reaction temperature was controlled at 35° C., and the reaction time was 4 hours. A yellow solid precipitated out during the addition, and then dissolved, and the solution turned into a yellow clear solution. The round bottom flask was then heated to reflux in an oil bath at 120°C for 6 hours. After cooling to room temperature, use 30% mass fraction sodium hydroxide solution to adjust the pH of the reaction solution to be neutral, add NaCl solid until the solution is saturated, extract the aqueous solution with isopropyl ether, separate the layers, take the organic layer, and remove most of it by distillation under reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com