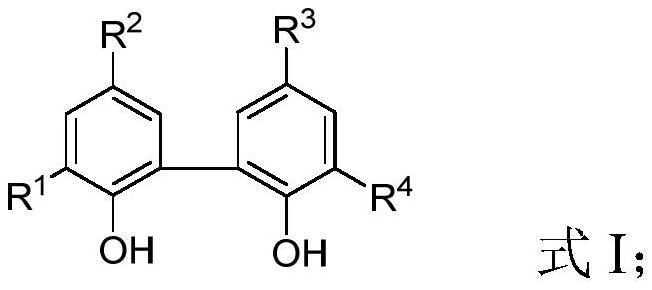
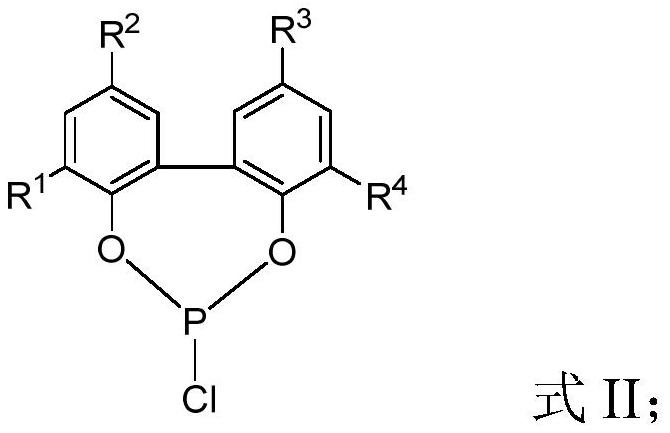
Purification method and application of 2, 2 '-biphenoxy phosphorus-chlorine compound
A technology of biphenoxy phosphorus chloride and purification method is applied in the field of chemical industry and can solve the problems of complex reaction system, low bisphosphite yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] This example provides a purification method for 2,2'-biphenoxyphosphorous chloride compounds, including:
[0080] The 50L glass reactor was replaced with nitrogen three times, phosphorus trichloride (10.00kg) was added at 0°C, 2,2′-biphenol (8.04kg) was added in batches while stirring, and stirring was continued for 15 hours to 2, 2'-biphenol was completely dissolved. Then heat up to reflux and continue to react for about 3 hours, then add triethylamine (1.09kg), dichloromethane (4L), toluene (6L) mixed solution, control the temperature to 80°C and steam under normal pressure to distill out the mixture containing phosphorus trichloride and Distill the mixture of hydrogen chloride for about 3 hours until no liquid flows out; then increase the temperature to 115°C and change to vacuum distillation, keep the vacuum at 50mbar and continue distillation for 2 hours, stop the distillation and cool to room temperature, brown viscous residual liquid in the reaction kettle It ca...
Embodiment 2
[0086] This example provides a purification method for 2,2'-biphenoxyphosphorous chloride compounds, including:
[0087] The 50L glass reactor was replaced with nitrogen three times, phosphorus trichloride (10.00kg) was added at -10°C, 2,2′-biphenol (8.04kg) was added in batches while stirring, and stirring was continued for 12 hours to 2 , 2′-biphenol was completely dissolved. Then raise the temperature to reflux and continue the reaction for about 3 hours, then add pyridine (1.35kg), 1,1-dichloroethane (5L), and xylene (5L) mixed solution, control the temperature to 70°C and steam under normal pressure Distill the mixture of phosphorus chloride and hydrogen chloride for about 3 hours until no liquid flows out; then raise the temperature to 125°C for vacuum distillation, keep the vacuum at 20mbar and continue distillation for 1.5 hours, stop the distillation and cool to room temperature, the brown viscous The thick residue can be used as a raw material for the synthesis of b...
Embodiment 3
[0093] This example provides a purification method for 2,2'-biphenoxyphosphorous chloride compounds, including:
[0094] The 50L glass reactor was replaced with nitrogen three times, phosphorus trichloride (7.20kg) was added at -5°C, 2,2′-biphenol (8.04kg) was added in batches while stirring, and stirring was continued for 30 hours to 2 , 2′-biphenol was completely dissolved. Then raise the temperature to reflux and continue to react for about 3 hours, then add imidazole (0.92kg), dichloromethane (2.2L), xylene (2.8L) mixed solution, control the temperature to 75°C and distill out the phosphorus trichloride containing Distill the mixture of hydrogen chloride and hydrogen chloride for about 3 hours until no liquid flows out; then increase the temperature to 125°C and change to vacuum distillation, keep the vacuum at 10mbar and continue distillation for 1 hour, stop the distillation and cool to room temperature, brown sticky residue in the reaction kettle The solution can be us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com