Method for preparing polycarbonate through catalysis of binuclear ionic liquid
A technology of ionic liquid and polycarbonate, which is applied in clean catalysis and green fields, can solve the problems that cannot be applied to the field of PC synthesis, cannot promote molecular chain condensation, and the catalyst has no reactivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
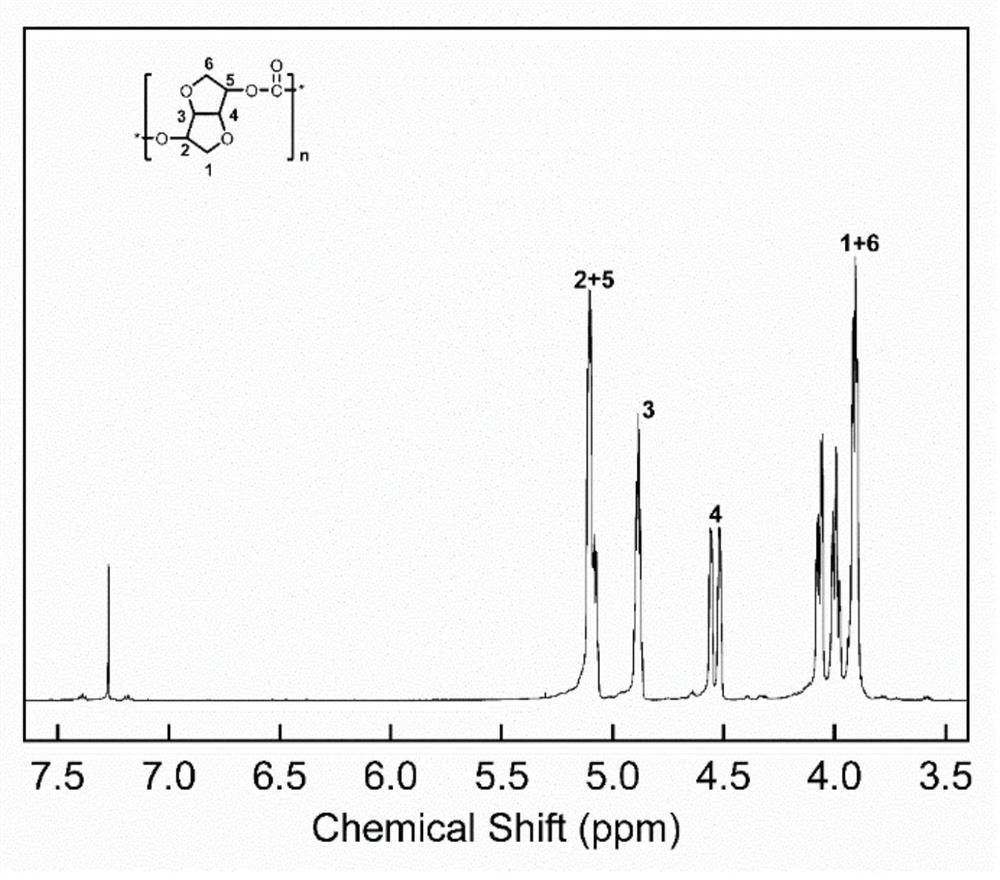
[0064] At room temperature, 10.70 g (50 mmol) of diphenyl carbonate (hereinafter referred to as "DPC") and 7.35 g (50 mmol) of isosorbide (hereinafter referred to as "ISO") were added to a 250 ml four-necked flask (ISO The molar ratio with DPC is 1:1), pass into nitrogen protection, heat up to 130°C and add catalyst bis-(3-methyl-1-imidazole)-ethylene bromide (isosorbide 1.5×10 moles of alcohol -5 ), the prepolymerization stage was kept at 130°C for 3h under normal pressure. The polycondensation stage reduces the pressure in the reactor to 1×10 -5 Mpa gradually raised the reaction temperature to 240° C., and continued the reaction for 15 minutes.
[0065] Concrete reaction formula is as follows, and the productive rate that obtains product A through calculation is 99%, and characterization analysis obtains that product A weight average molecular weight is 89800g / mol, and glass transition temperature is (T g )173℃, thermal decomposition temperature is (T d5% ) 328°C.
[00...
Embodiment 2
[0068] Same as Example 1, except that the catalyst is bis-(3-methyl-1-imidazole)-propylene bromide, other conditions remain unchanged. The productive rate that obtains product A through calculation is 97%, and characterization analysis obtains product A weight-average molecular weight to be 82600g / mol, T g 172°C, T d5% is 325°C.
Embodiment 3
[0070] Same as Example 1, except that the catalyst is bis-(3-methyl-1-imidazole)-butylene bromide, other conditions remain unchanged. The productive rate that obtains product A through calculation is 98%, and characterization analysis obtains product A weight-average molecular weight to be 84500g / mol, T g 169°C, T d5%It is 321°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com