Method for preparing mesitylene from carbon monoxide and methanol
A carbon monoxide and durene technology, applied in chemical instruments and methods, hydrocarbon production from oxygen-containing organic compounds, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as restricting the production scale of durene, Achieve good industrial application prospects, simple production process, and good catalyst stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
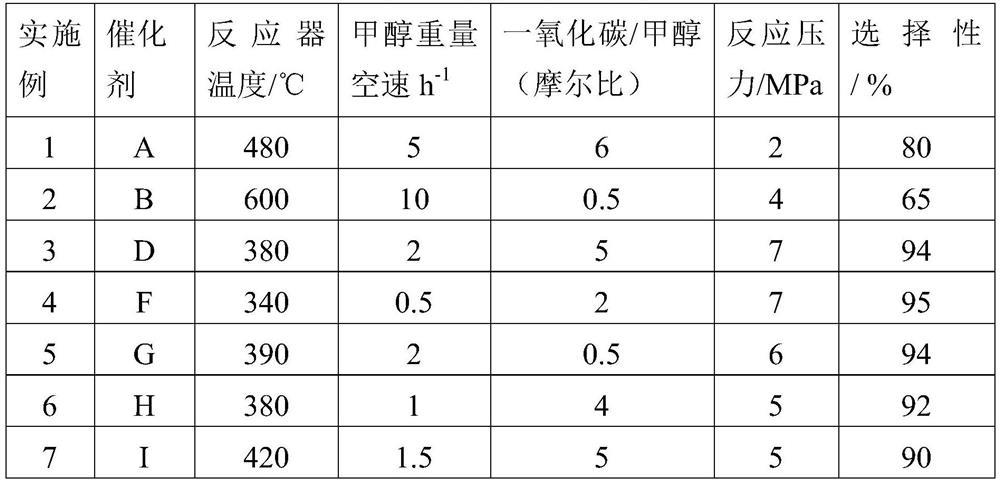
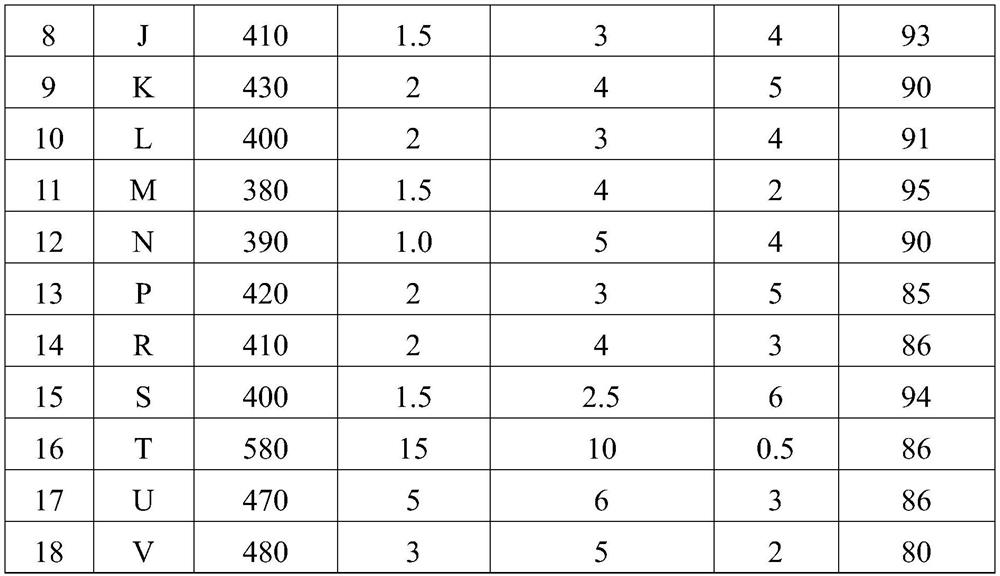
Examples
Embodiment 1
[0098] The preparation process of the catalyst is as follows: 120 grams of HZSM-5 molecular sieve with a molar ratio of silica to aluminum of 20 is mixed with 60 grams of diatomaceous earth and 100 grams of silica sol with a weight content of 20% silica, and an appropriate amount of 10% dilute nitric acid is added as an auxiliary agent. Extrusion molding. Dry at 120°C and bake at 500°C for 10 hours. The above catalyst was cut into 1 mm to obtain a columnar catalyst precursor A0 (ie, the intermediate product I).
[0099] The A0 sample of 20 grams adopts the aqueous solution dipping that contains copper nitrate, zinc nitrate simultaneously for 12 hours, 120 ℃ of oven dry, 600 ℃ of roasting 3 hours, copper oxide weight content is 3%, zinc oxide weight content 3%, obtained A1 ( Namely intermediate product II).
[0100] A2 (ie intermediate product III) was obtained by calcining 20 grams of A1 in a 100% water vapor atmosphere for 10 hours at a temperature of 350°C, a pressure of 1...
Embodiment 2
[0103] The preparation process of the catalyst is as follows: 60 grams of HZSM-5 molecular sieve with a molar silicon-alumina ratio of 30 is mixed with 100 grams of silica sol with a weight content of 40% silica and 100 grams of alumina, and an appropriate amount of 10% dilute nitric acid is added as an auxiliary agent to extrude bars. forming. Dry at 120°C and bake at 700°C for 4 hours. The above catalyst was cut into 3 mm to obtain a columnar catalyst precursor B0.
[0104] A 20-gram B0 sample was soaked in an aqueous solution containing both ferric nitrate and zinc nitrate for 12 hours, dried at 120°C, and calcined at 550°C for 10 hours. The iron oxide weight content was 15% and the zinc oxide weight content was 1% to obtain B1.
[0105] B2 was prepared by calcining 20 grams of B1 in a 100% water vapor atmosphere for 0.5 hours at a temperature of 800 °C, a pressure of 2.0 MPa, and calcination at 600 °C for 3 hours.
[0106] To 20 grams of B2, add 50 ml of 1% phosphoric ac...
Embodiment 3
[0108] The preparation process of the catalyst is as follows: 200 grams of HZSM-5 molecular sieve with a molar silicon-alumina ratio of 40 is mixed with 20 grams of diatomite and 30 grams of kaolin, and an appropriate amount of 10% dilute nitric acid is added as an auxiliary for extrusion molding. Dry at 120°C and bake at 550°C for 4 hours. The above catalyst was cut into 1.5 mm to obtain a columnar catalyst precursor D0.
[0109] The D0 sample of 20 grams was impregnated with an aqueous solution containing both zinc nitrate and cerium nitrate for 24 hours, dried at 120 ° C, and calcined at 600 ° C for 3 hours, the weight percentage of zinc oxide was 8%, and the weight percentage of cerium oxide was 1% to obtain D1 .
[0110] D2 was prepared by calcining 20 grams of D1 in a 100% water vapor atmosphere for 10 hours, at a temperature of 350°C, a pressure of 3.0 MPa, and calcination at 650°C for 3 hours.
[0111] 20 grams of D2 was added to 50 ml of 5% nitric acid solution by w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com