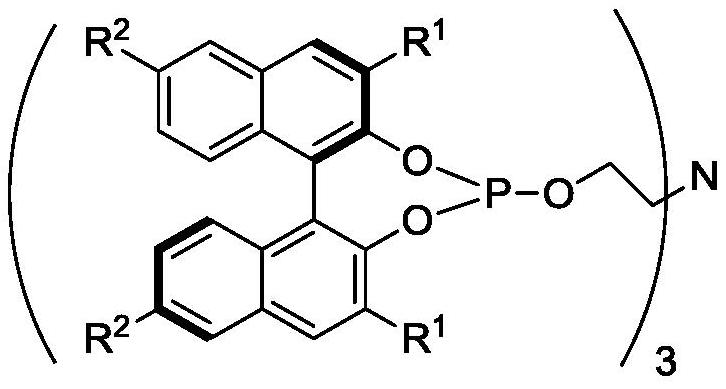
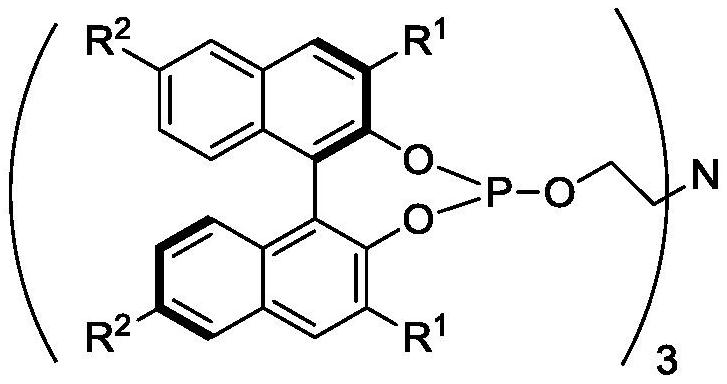
Chiral tetradentate ligand, chiral ruthenium complex and method for preparing (R)-(-)-1, 3-butanediol
A ruthenium complex and chiral technology is applied in the field of asymmetric hydrogenation of chiral ligands and the preparation of 1,3-butanediol, which can solve the problems of large amount of catalyst, difficult product separation and high cost, and achieves The reaction operation is simple, the raw materials are cheap and easy to obtain, and the preparation is simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
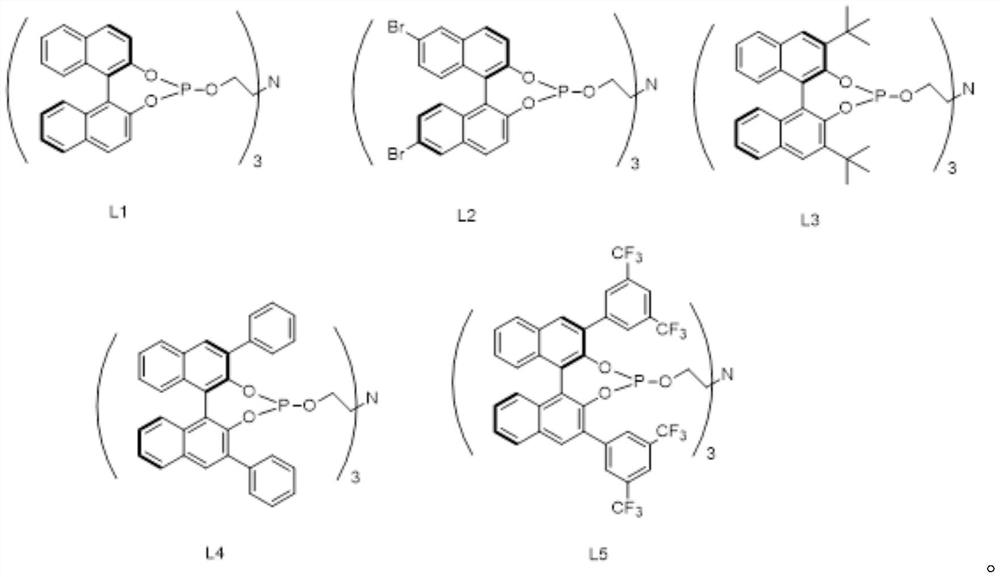
[0064] Example 1: Synthesis of chiral tetradentate phosphine nitrogen ligand L1
[0065] In the glove box, add (R)-1,1'-bi-2-naphthol (1g, 3.49mmol), DMAP (0.004g, 0.035mmol) and triethylamine (10g, 0.1mol) into a 50mL Schlenk bottle , It was sealed; Out of the glove box, put the Schlenk bottle into the water bath, start stirring, add phosphorus trichloride (0.48g, 3.49mmol) to the Schlenk bottle under argon atmosphere, and stir at room temperature for 1h. Continue to add triethanolamine dropwise to the Schlenk bottle under an argon atmosphere. After the dropwise addition, put the Schlenk bottle into an oil bath and heat to reflux for 2 to 3 hours. After the complete reaction of the raw material dinaphthol is monitored by TLC, the reaction is terminated. The reaction solution was cooled to room temperature, and the base was recovered by distillation under reduced pressure to obtain a crude tetradentate phosphine nitrogen ligand. The crude product was crystallized with a mixed...
Embodiment 2
[0066] Example 2: Synthesis of chiral tetradentate phosphine nitrogen ligand L2
[0067] In the glove box, add (R)-6,6'-dibromo-1,1'-bi-2-naphthol (0.5g, 1.125mmol), DAMP (1.4mg, 0.011mmol) and Triethylamine (10g) is sealed; out of the glove box, the Schlenk bottle is put into a water bath, and the stirring is started, and phosphorus trichloride (0.17g, 1.24mmol) is added to the Schlenk bottle under an argon atmosphere, and Stir for 1h. Continue to add triethanolamine (67mg, 0.45mmol) dropwise to the Schlenk bottle under the argon atmosphere. After the dropwise addition, the Schlenk bottle is put into the oil bath, and heated to reflux for 3h. After TLC monitors that the raw material dinaphthol has completely reacted, Stop the reaction. The reaction solution was cooled to room temperature, and the base was recovered by distillation under reduced pressure to obtain a crude tetradentate phosphine nitrogen ligand. The crude product was crystallized with a mixed solvent of dich...
Embodiment 3
[0068] Example 3: Synthesis of chiral tetradentate phosphine nitrogen ligand L3
[0069] In the glove box, add (R)-3,3'-di-tert-butyl-1,1'-bi-2-naphthol (0.2g, 0.50mmol), DAMP (0.61mg, 0.0050mmol) into a 10mL Schlenk bottle ) and triethylamine (6g), which are sealed; out of the glove box, the Schlenk bottle is put into a water bath, and the stirring is started, and phosphorus trichloride (76mg, 0.55mmol) is added to the Schlenk bottle under an argon atmosphere, and room temperature Stir for 1h. Continue to add triethanolamine (30mg, 0.20mmol) dropwise to the Schlenk bottle under an argon atmosphere. After the dropwise addition, the Schlenk bottle is put into an oil bath, and heated to reflux for 3h. After TLC monitors that the raw material dinaphthol has completely reacted, Stop the reaction. The reaction solution was cooled to room temperature, and the base was recovered by distillation under reduced pressure to obtain a crude tetradentate phosphine nitrogen ligand. The cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com