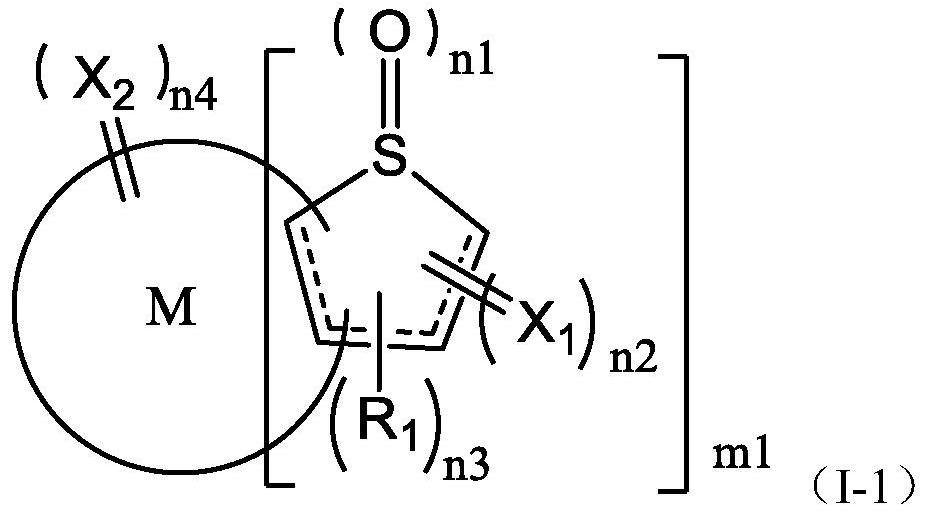
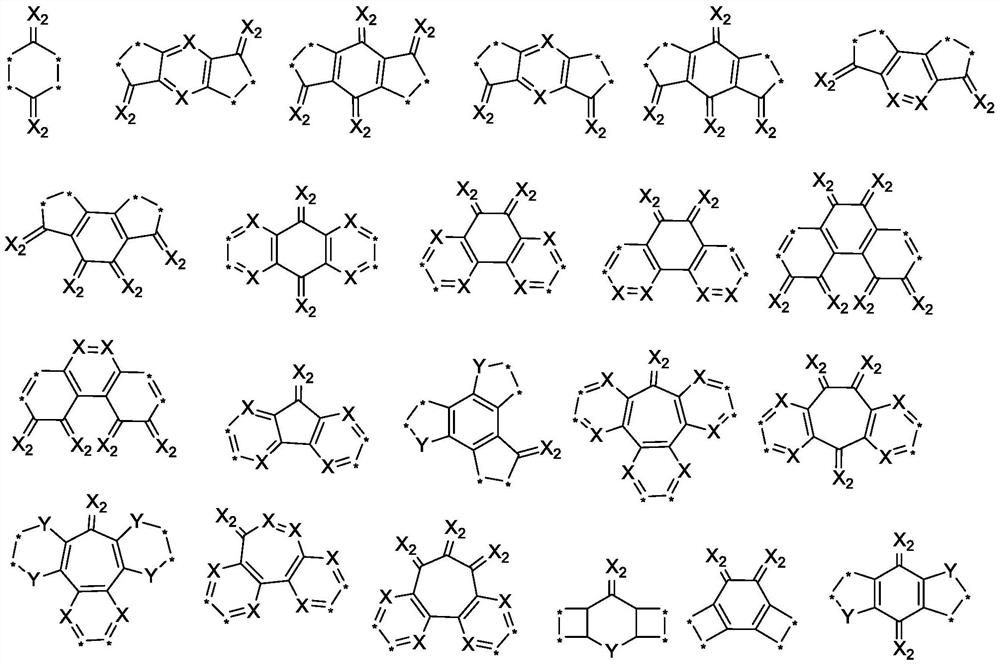
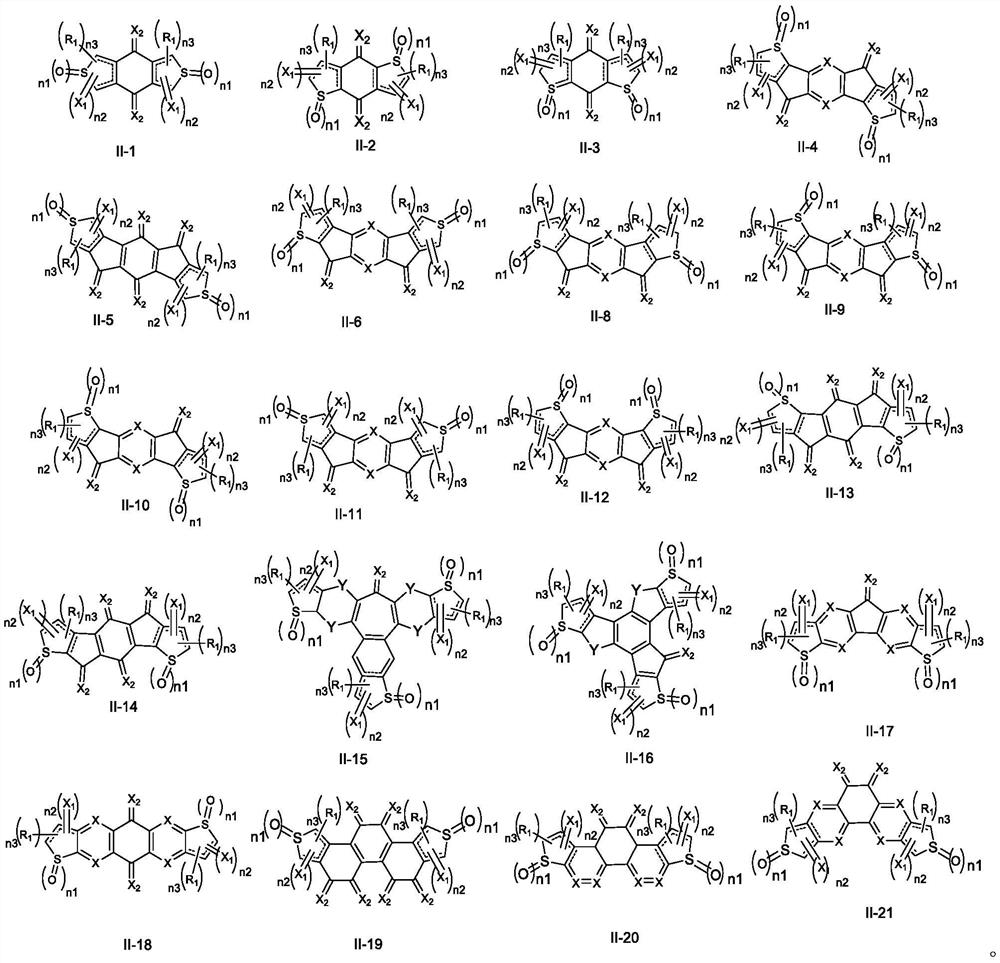
Fused ring compound containing thiophene oxide and application thereof
A technology of compound and thiophene, which is applied in the field of organic electroluminescence, can solve problems such as insufficient stability, reduced lifetime of organic light-emitting diodes, and reduced lifetime, achieve excellent hole transport properties and stability, improve electroluminescence efficiency, The effect of prolonging life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0148] The synthesis method of the compound of the present invention is exemplified, but the present invention is not limited to the following examples.
[0149] 1. Compounds and synthetic steps
[0150]
Embodiment 1
[0151] Embodiment 1: the synthesis of compound PD-1
[0152]
[0153] (1) Synthesis of intermediate 2:
[0154] Dissolve compound 1 (1mmol) and propanedicyanide (3.5mmol) in 50mL of anhydrous chloroform, then add 4mL of titanium tetrachloride and 4mL of pyridine, stir and reflux under nitrogen protection for 24h, add ice water after the reaction, and precipitate After filtration, 132 mg of intermediate 2 was obtained with a yield of 42%.
[0155] (2) Synthesis of Compound PD-1:
[0156] Add 30% hydrogen peroxide (0.3ml, 5.35mmol) in a 100mL three-necked flask, add trifluoroacetic anhydride (0.6ml, 4.25mmol) dropwise under ice-cooling, stir for 30min, then add intermediate 2 (111mg, 0.35mmol) dropwise ) in dichloromethane solution 15ml, reacted overnight. Slowly pour the reaction solution into ice water, adjust the pH value to 9 with sodium bicarbonate, extract with ethyl acetate three times, wash with water three times, dry over anhydrous magnesium sulfate, separate and ...
Embodiment 2
[0158] Embodiment 2: the synthesis of compound PD-2
[0159]
[0160] (1) Synthesis of intermediate 5:
[0161] Add compound 3 (10 mmol), compound 4 (20 mmol), 15 mL of saturated aqueous sodium carbonate solution, 160 mg of triphenylphosphine, 80 mg of palladium acetate, and 80 mL of THF into the two-necked flask in turn, and pass nitrogen gas for 30 minutes, and the system is heated to 66 ° C. Reflux reaction overnight, add water to terminate the reaction, extract with EA and brine, add anhydrous magnesium sulfate to the EA phase to dry, then suction filter, and pass the filtrate through a silica gel column to obtain 3.36 g of intermediate 5 with a yield of 87%.
[0162] (2) Synthesis of Intermediate 6:
[0163] Add intermediate 5 (10mmol), KOH 23g, water 100mL, EtOH 230mL, stir, react overnight at 78°C, after the reaction, spin dry the alcohol, add hydrochloric acid to neutralize to acidity, and extract with EA to obtain 3.23g of intermediate 6. The rate is 98%.
[016...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com