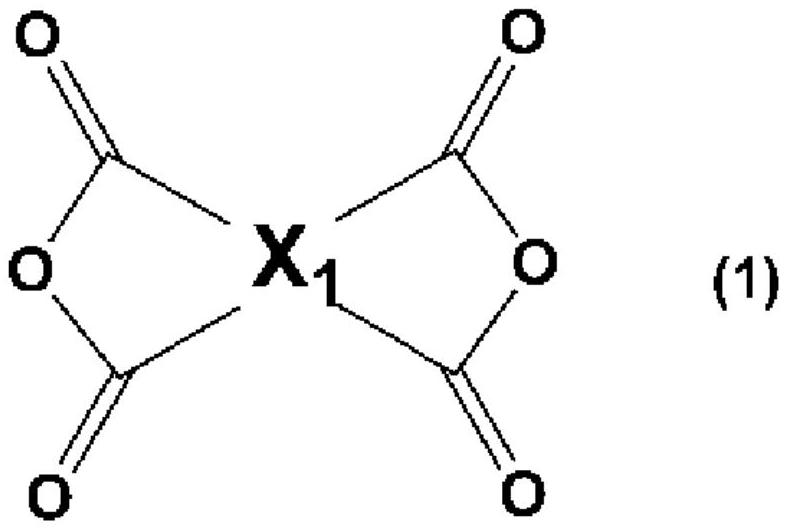
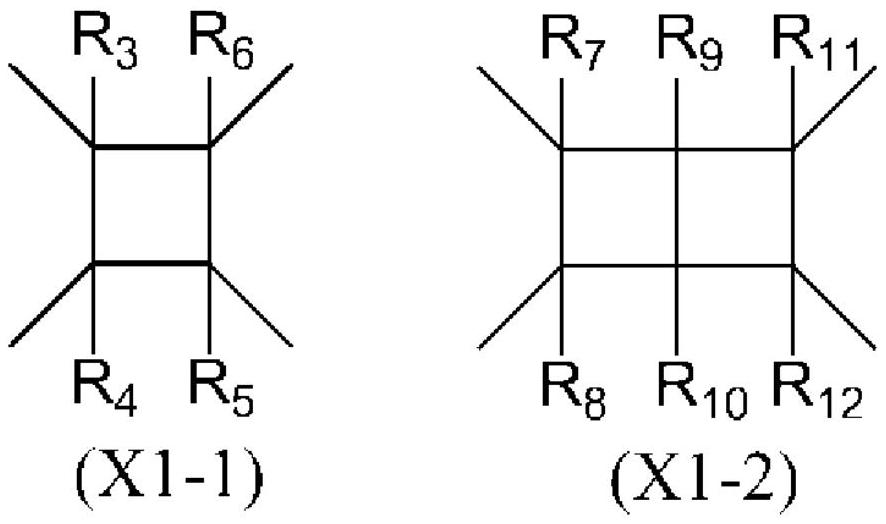
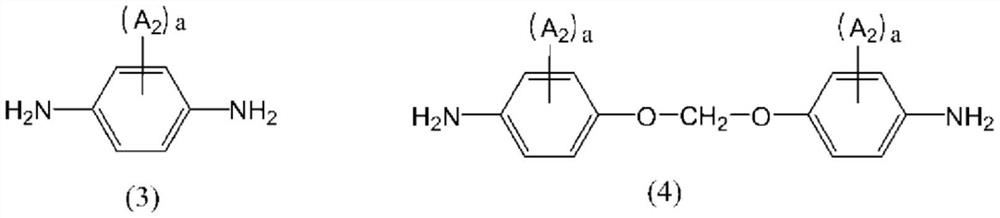
Liquid crystal alignment agent, production method thereof, liquid crystal alignment film, and liquid crystal display element
A technology of liquid crystal alignment agent and liquid crystal alignment film, which is applied in the fields of instruments, optics, nonlinear optics, etc., can solve the problems of increasing the manufacturing process of liquid crystal display elements, and achieve excellent afterimage characteristics, high yield, and reduced light exposure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0117] Although an Example is given to the following and this invention is demonstrated more concretely, this invention is not limitedly interpreted here.
[0118] The abbreviations of the compounds in the following and the measurement methods of each characteristic are as follows. It should be noted that the numerical values and units in the following are mass standards unless otherwise mentioned.
[0119] NMP: N-methyl-2-pyrrolidone, GBL: γ-butyrolactone,
[0120] BCS: butyl cellosolve,
[0121]
[0122] However, in the above formula, Boc represents a tert-butoxycarbonyl group.
[0123]
[0124] [viscosity]
[0125] Using an E-type viscometer TVE-22H (manufactured by Toki Sangyo Co., Ltd.), it was measured with a sample volume of 1.1 mL, a cone rotor TE-1 (1°34', R24), and a temperature of 25°C.
[0126] [molecular weight]
[0127] It measured using a GPC (ordinary temperature gel permeation chromatography) apparatus, and calculated Mn and Mw as polyethylene gly...
Synthetic example 2~6
[0148] Using the diamine component, the tetracarboxylic acid component, and NMP shown in the following Table 1, and implementing at the reaction temperature, it was carried out in the same manner as in Synthesis Example 1 to obtain the polymer shown in the following Table 1. Amic acid solutions (A-2) to (A-4), (B-1), and (B-2). The viscosity and Mn / Mw of the obtained polyamic acid are shown in Table 1 below.
[0149] In addition, the density|concentration of the polyamic acid in the polyamic-acid solution obtained by the synthesis examples 1-6 was all 15 mass %.
[0150] [Table 1]
[0151]
[0152]
[0153] 50 g of NMP was added to 100 g of the obtained polyamic acid solution (A-1) in a 300 mL four-neck flask equipped with a stirring device and a nitrogen gas introduction tube, and stirred for 30 minutes. 17.60 g of acetic anhydride and 5.50 g of pyridine were added to the obtained polyamic acid solution, and it heated at 50 degreeC for 3 hours, and chemically imidized ...
Synthetic example 8~10
[0157] Except using acetic anhydride and pyridine shown in the following Table 2, the chemical imidization operation was carried out in the same manner as in Synthesis Example 7 to obtain the polyimide solution (A- 2-PI) ~ (A-4-PI). The imidization rate and Mn / Mw of the obtained polyimide are shown in Table 2 below.
[0158] [Table 2]
[0159]
[0160]
[0161] Take 4.0 g of 12 mass % polyimide solution (A-1-PI) obtained in Synthesis Example 7 and 4.8 g of 15 mass % polyamic acid solution (B-1) obtained in Synthesis Example 5 to 50 ml NMP 3.24g, GBL 3.96g, and BCS4.00g were added to the Erlenmeyer flask, and it mixed at 25 degreeC for 8 hours, and obtained the liquid crystal aligning agent (1) (refer following Table 3). Abnormalities such as turbidity and precipitation were not seen in this liquid crystal aligning agent, and it was confirmed that it was a uniform solution.
[0162] In addition, in Table 3, A / B shows the mass % ratio of polyimide solution / polyamic acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com