Organic compound and preparation method and application thereof
An organic compound and condensation reaction technology, applied in organic chemistry, sulfide preparation, flotation, etc., can solve the problems of poor solubility, poor short-chain hydrophobicity of amido hydroxamic acid, etc., and achieve easy operation and good flotation Effect, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
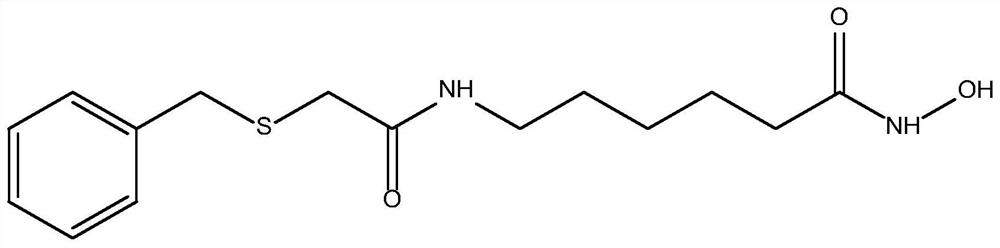
[0047] Embodiment 1: the preparation of N-benzylthioacetylaminohexyl hydroxamic acid
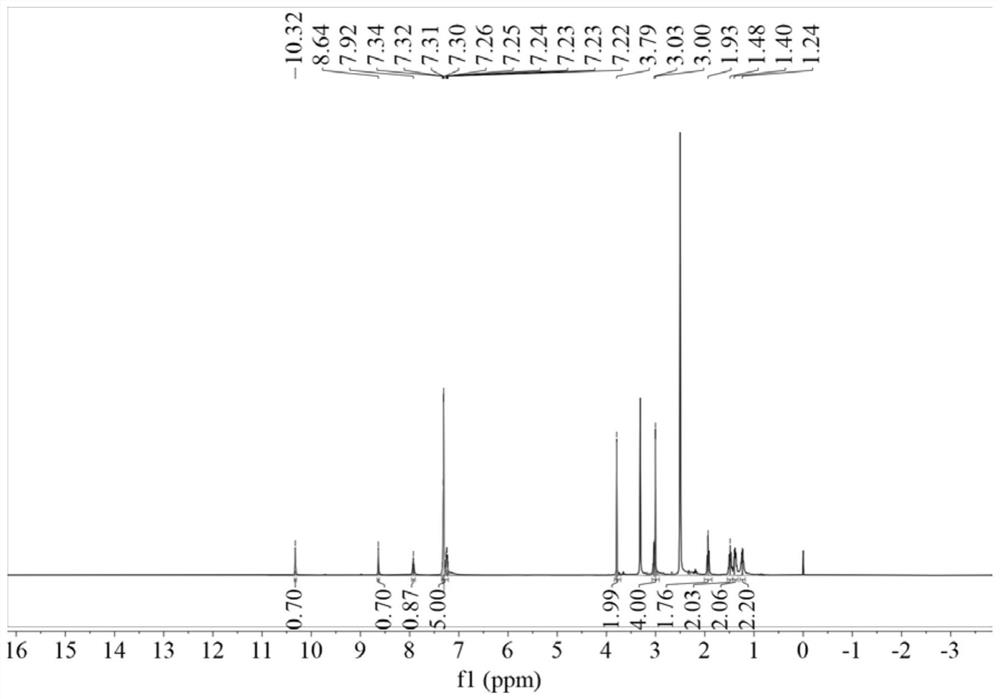
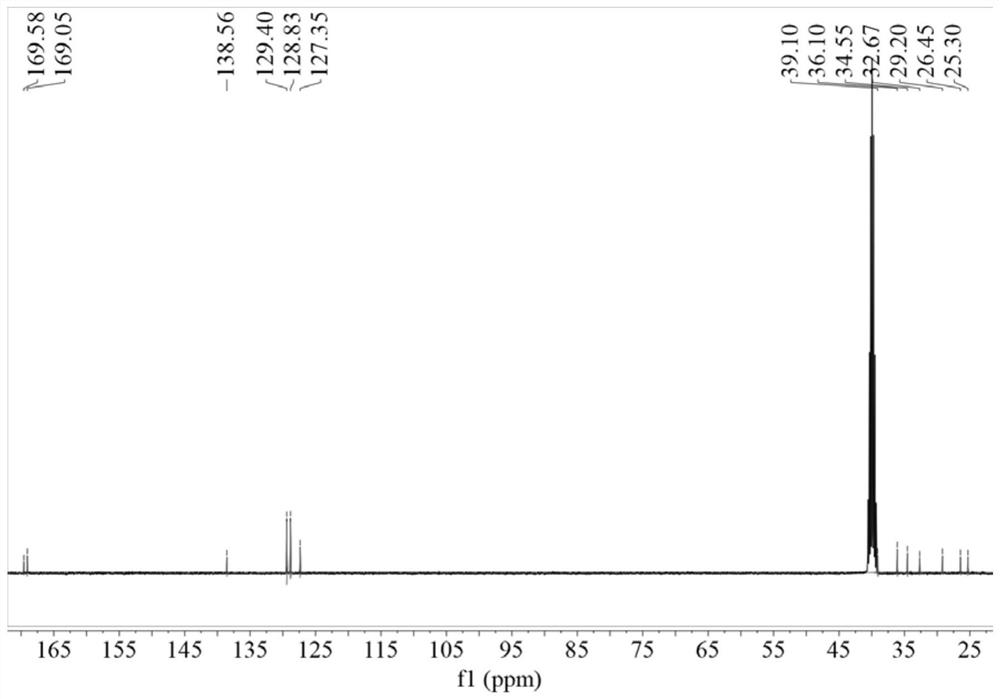
[0048] Weigh 3.64g 96.15% benzylthioacetic acid into 50ml dichloromethane, add 3.24g 99% 1,1'-carbonyldiimidazole and 3.63g 98% 6-aminocaproic acid methyl ester hydrochloride, room temperature React under the conditions for 6 hours, after the reaction is finished, distill under reduced pressure, wash with water, and dry to obtain methyl N-benzylthioacetylaminocaproate; weigh 2.94g of 96% sodium hydroxide and slowly add it dropwise to 2.68g of 99.5% hydroxylamine hydrochloride Stir in the solution for 0.5h; weigh 10.82g of N-benzylthioacetylaminocaproic acid methyl ester, add the mixed solution of hydroxylamine hydrochloride and sodium hydroxide, heat to 40°C under stirring, and react for 4h to obtain the desired N-benzyl The thioacetylaminohexyl hydroxamic acid product has the structure figure 1 Shown; The optimal configuration at DFT / B3LYP 6-311G(d) level is as follows Figure 5 Shown; DF...
Embodiment 2
[0056] Embodiment 2: the preparation of N-benzylthioacetylaminohexyl hydroxamic acid
[0057] Weigh 18.20g of 96.15% benzylthioacetic acid into 50ml of dichloromethane, add 16.20g of 99% 1,1'-carbonyldiimidazole and 18.15g of 98% 6-aminocaproic acid methyl ester hydrochloride, room temperature Under the conditions of reaction for 10 hours, after the reaction was completed, distilled under reduced pressure, washed with water, and dried to obtain methyl N-benzylthioacetamide caproate; weighed 4.20g of 96% sodium hydroxide and slowly added dropwise to 3.82g of 99.5% hydroxylamine hydrochloride Stir in the solution for 0.5h; weigh 15.45g of N-benzylthioacetylaminocaproic acid methyl ester, add the mixed solution of hydroxylamine hydrochloride and sodium hydroxide, heat to 40°C under stirring, and react for 4h to obtain the desired N-benzyl Thioacetamidohexyl hydroxamic acid product. The yield based on benzylthioacetic acid was 76.21%.
Embodiment 3
[0059] Embodiment 3: Iron ion in N-benzylthioacetylaminohexyl hydroxamic acid analysis solution
[0060] Mix 50ml of 1% N-benzylthioacetylaminohexyl hydroxamic acid solution with 10ml of Fe of unknown concentration 3+ The solution was mixed and shaken in a constant temperature oscillator at 30°C for 10 minutes. By measuring the absorbance in the aqueous phase and the absorbance of the original N-benzylthioacetylaminohexyl hydroxamic acid solution, it can be known that Fe 3+ The concentration is 0.12g / L. It can be seen that N-benzylthioacetylaminohexyl hydroxamic acid can be used as an analytical reagent for iron ions, and compared with o-phenanthroline, a common analytical reagent for iron ions, it is less toxic and more environmentally friendly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com