Application of heat-resistant alpha-glucuronidase polypeptide fusion body in preparation of glucuronic acid
A technology of glucuronidase and glucuronic acid, applied in the field of glucuronic acid, can solve problems such as waste of resources and environmental pollution, and achieve the effects of less reaction by-products, large amount of products and improved activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] According to the α-glucuronidase gene sequence (the accession number of the GenBank database of Ncbid: GeneID:896879) of the family 67 of Thermotoga (Thermotiga), namely the nucleotide sequence design primer of coding wild-type TmAguA, specifically:
[0039] upstream primer
[0040] F: 5'CATG CCATGG ACTACAGGATGTGCTGGCT-3' (SEQ ID NO.3)
[0041] Downstream primers (four types)
[0042] R1: 5'CCG CTCGAG CGGATATATCTTTCTTTCCCTT-3' (SEQ ID NO.4)
[0043] R2: 5'CCG CTCGAG TGCATGAAAGAATGCCTGATGCGGATATATCTTCTTCCC-3' (SEQ ID NO. 5)
[0044] R3: 5'CCG CTCGAG GCCGCTGCCGCTGCCCGCCGCCGGATATATCTTCTTCCC-3' (SEQ ID NO. 6)
[0045] R4: 5'CCG CTCGAG GCCGCCGCTGCCGCCGCCGCCCGGATATATCTTCTTCCC-3' (SEQ ID NO. 7)
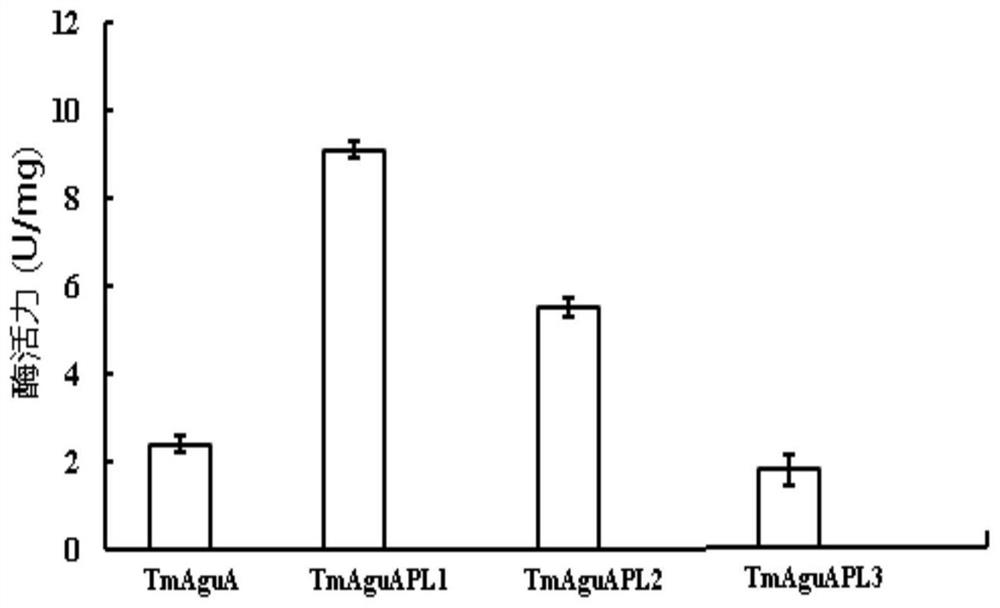
[0046] The recombinant plasmid pET-28a-xynB-Hspr-aguA (J.Carbohydrate Chemistry,2018,37(4)210-224.) containing the wild-type α-glucuronidase gene of Thermotoga maritima was selected as a template, respectively Four pairs of primers F and R1 (wild-type TmAguA), F and R2 ...
Embodiment 2
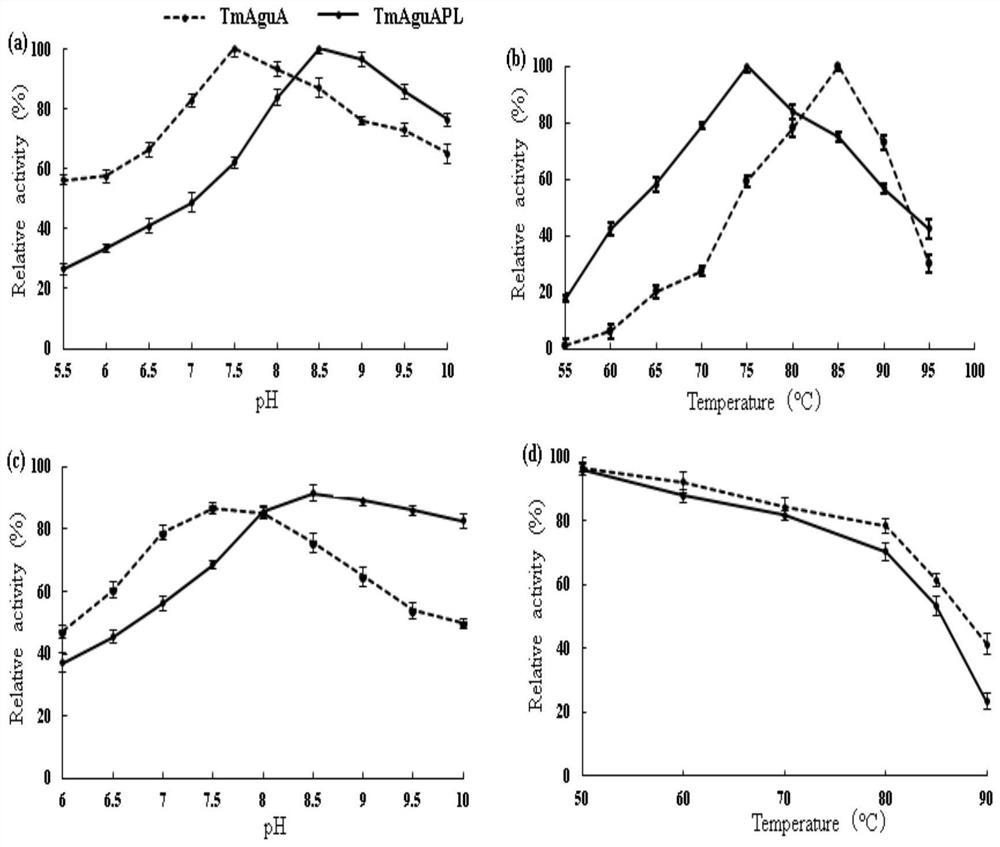
[0048] Using the same method as in Example 1 to obtain purified TmAguA and TmAguAPL1, further compare the effects of pH and temperature on the enzyme activity of TmAguA and TmAguAPL1:
[0049] (1) Optimum reaction pH: place the recombinant enzymes TmAguA and TmAguAPL1 at 75°C to determine the effect of different pH on the enzyme activity, take the highest enzyme activity as 100%, calculate the relative activity of the enzyme, and make a relative activity-pH curve. The enzyme reaction was carried out in 50 mM o-benzyme buffer (pH 5.5-10.0), and the pH of the buffer was adjusted at room temperature. The above determination was repeated three times, and the relative standard deviation was less than 10%.
[0050] (2) pH stability: place the recombinant enzymes TmAguA and TmAguAPL1 in a buffer solution with a pH value of 5.5-10 and incubate at 37°C for 1 hour, then measure the residual activity of the enzyme. Relative activity, for pH stability curve. The buffer used was 50 mM o-be...
Embodiment 3
[0058] The method is the same as in Example 2, except that equimolar amounts of TmAguA and TmAguAPL1 are further selected to evaluate the effect of enzymatically hydrolyzing xylan to prepare glucuronic acid. The specific method:
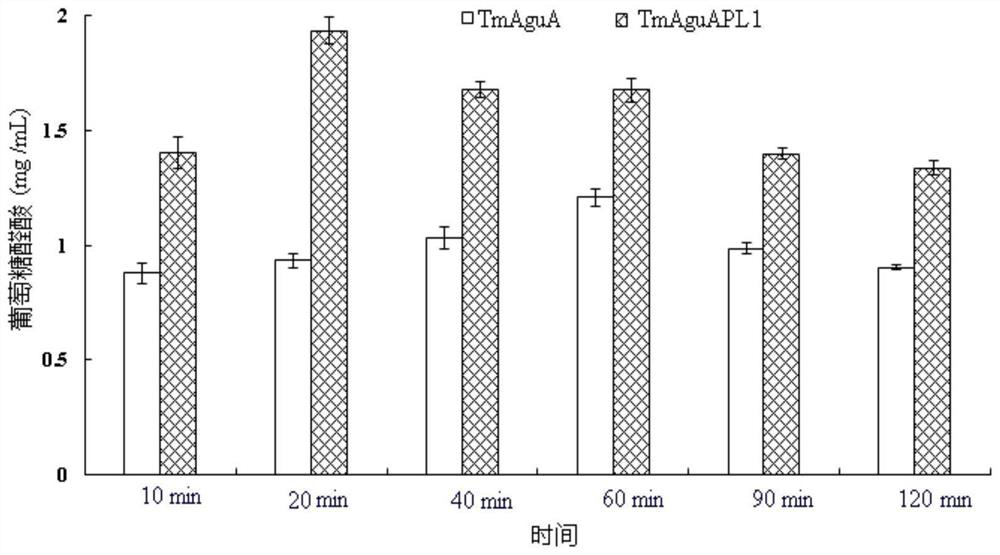
[0059] Beech wood xylan was dissolved in phosphate buffer solution to prepare a beech wood xylan solution with a concentration of 10 mg / mL, and 20 U / g (substrate) of β-1,4-endoxylanase was added, and then Add 1.82 μmol / L of purified TmAguA and incubate at 85°C and pH7.5 for 2 hours as a control group; add purified TmAguAPL1 with equimolar concentration (1.82μmol / L) and incubate at 75°C and pH8.5 for 2 hours, Results (see image 3 ) shows that: compared to the control group (wild-type enzyme), the sample group (TmAguAPL1) converts beech wood xylan to produce glucuronic acid production increased by 48.6-60.3%, and the gained TmAguAPL1 is hydrolyzed 4-O-methylglucuronic acid xylan The sugar releases the highest glucuronic acid, its nucleic acid sequenc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com