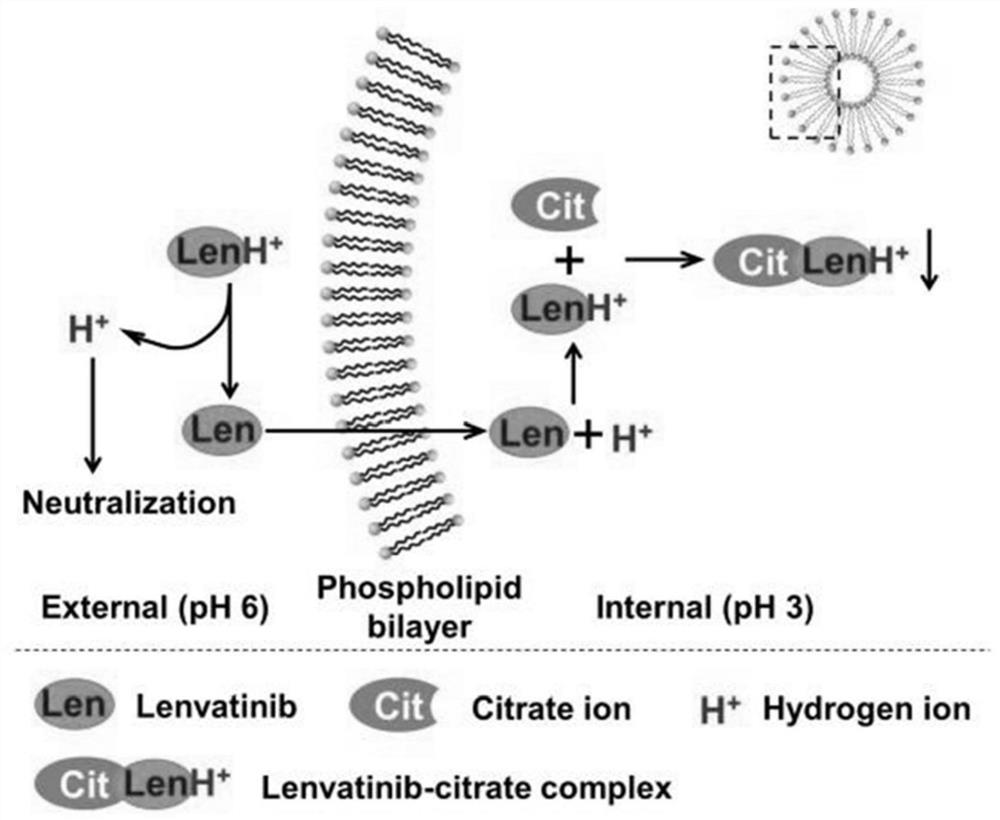
Lenvatinib liposome, pharmaceutical composition thereof, preparation method thereof and prescription process optimization method
A lenvatinib and liposome technology, applied in the field of medicine, can solve the problem of low bioavailability of lenvatinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
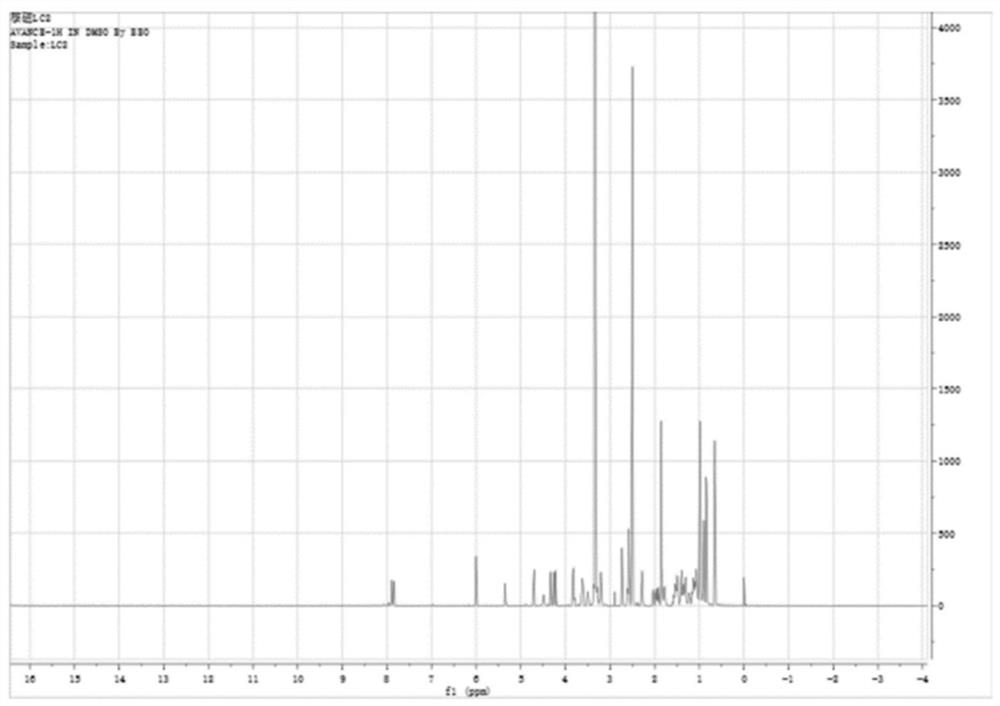
[0040]Example 1 Sialic acid - Tenxin - stearate (SA-PG10-C18Synthesis and characterization
[0041]SA-PG according to the present invention10-C18Preparation process: First, the proportion of the feed is sialic acid (SA) / ten polyglycerol - stearate (Pg10-C18) / N-hydroxybutyimide (NHS) / 1-ethyl-(3-dimethylaminopropyl) carbonitidinimide hydrochloride (EDC) / triethylamine (TEA) = 2: 1: 2: 4: 4 (molar ratio); Sa is dissolved in 5 mL of formamide (FA), adding EDC / NHS, activation of EDC / NHS, at 4 ° C for 90min, at this time, the system is clarified; then dissolved in 2 ml of FA PG in PG10-C18In the addition of the reaction system, TEA was added, and the reaction was stirred at room temperature for 48 h, and the reaction solution was clarified; then the reaction solution was transferred to the dialysis bag (intercept molecular weight 10 kDa), and the dialysis medium was concentrated hydrochloric acid (V / V, 1: 100) system, The dialysis volume is 1000 ml dialysis, and the dialysis med...
Embodiment 2
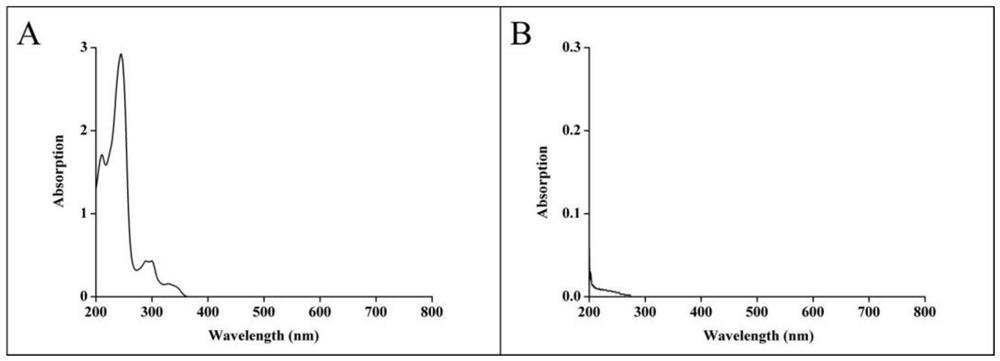
[0045]Example 2LEN UV detection wavelength determination and standard curve
[0046]First, it is prepared by isopropanol / water (90 / 10 V / V) to formulate the Laut Dibini (LEN) drug solution, the drug concentration is 0.1 mg / ml. Then, with isopropanol / water (90 / 10 V / V) as a white solvent, 200 to 800 nm full-wavelength scanning is performed with a UV2700 ultraviolet spectrophotometer to 0.1 mg / ml of a Len drug solution, and the optimum Len ultraviolet detection wavelength is determined.
[0047]Precision, 10.0 mg of Len raw materials, placed in a 100 mL volumetric flask, dissolved in isopropanol / water (90 / 10V / V) and diluted to the scale, and manufactured by 100 μg · ml of mass concentration-1LEN solution as a reserve. Squeezing the stock solution 0.5, 1.0, 2.0, 3.0, 4.0 ml, respectively, in a 10 ml volumetric flask, diluted with isopropanol / water (90 / 10 V / V) to the scale, i.e., the mass concentration is 5.0, 10.0, respectively. 20.0, 30.0, 40.0 μg · ml-1Len series standard...
Embodiment 3
[0050]Example 3 Method for preparing a lug niterni
[0051]The Len Liposomes according to the present invention are prepared by the classic liposome, and their groups are HSPC / CH / SA-PG.10-C18 / LEN = 100 mg / 33 mg / 33 mg / 5 mg, 10 ml of liposomes were prepared. Precision-priced placed HSPC, CH and SA-PG10-C18Placed in a 500 ml round bottom flask, 10 ml of anhydrous ethanol, completely dissolved HSPC, CH and SA-PG10-C18Thereafter, the water bath was heated at 65 ° C, the rotation was 30 rpm, and the vacuum was 0.09 MPa conditions, the water-evaporation was removed, a lipid film was formed on the round bottom flask; then, the precision weighed 5 mg LEN, completely dissolved in 10 ml In aqueous solution containing 10% ethanol, the Len solution was added to the round bottom flask, and the lipid film was 30 min under a rotational speed of 30 rpm. The lipid film was hydrated at room temperature atmospheric pressure; dispersed the liposomes using ultrasonic cell pulverizer, using 100W d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com