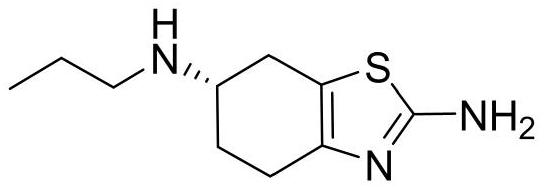
Preparation method of high-purity pramipexole
A high-purity, solvent-based technology, applied in the field of preparation of high-purity pramipexole, can solve the problems of low yield, high price, and high production cost, and achieve the effects of simplifying the preparation process, reducing costs, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
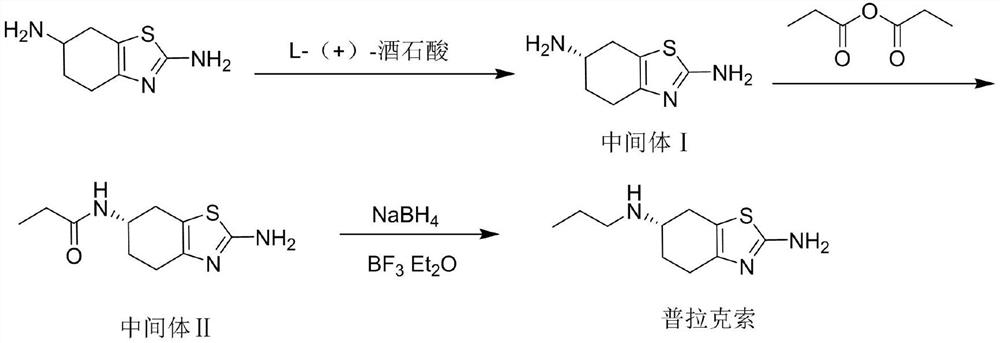
[0031] The invention provides a kind of preparation method of high-purity pramipexole, comprises the following steps:
[0032] (1) 2,6-diamino-4,5,6,7-tetrahydrobenzothiazole, the first solvent and L-(+)-tartaric acid are mixed for a resolution reaction to obtain a product liquid; After cooling down and crystallizing the product liquid, filter and dry in sequence, recrystallize the obtained crystalline product in the first solvent, mix the recrystallized product and the first solvent, add hydrochloric acid until the system dissolves, and use lye to adjust the pH of the system to The value is adjusted to ≥13, followed by filtration and drying to obtain (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole;
[0033] (2) Mix the (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole, the second solvent, triethylamine and propionic anhydride for condensation reaction, after the reaction is completed, Use ammonia water to quench the reaction, concentrate the obtained product material liquid, mix...
Embodiment 1
[0060] (1) Synthesis of (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (Intermediate I):
[0061]Add 2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (100.14g, 0.59mol) into 1500mL of purified water, heat up to 75°C under stirring, add L-(+)-tartaric acid ( 133.24g, 0.89mol), the system was dissolved, kept for 2 hours, cooled to 25°C, stirred and crystallized for 4 hours, filtered with suction, dried, added 1500mL of purified water to the dried product, heated to 77°C with stirring, the system was dissolved, kept warm React for 2 hours, cool down to 25°C, stir and crystallize for 4 hours, suction filter, dry, add 340 mL of purified water to the dry product, add concentrated hydrochloric acid dropwise under stirring until the system dissolves, adjust the pH to ≥13 with 50wt.% NaOH solution, pump Filter and dry to obtain 48.5 g of off-white solid (intermediate I), the yield is 48.4%, HPLC shows that the purity is 99.95%, isomer detection: more than 99.5%;
[0062] (2) Synthesis of ...
Embodiment 2
[0069] (1) Synthesis of (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (Intermediate I):
[0070] Add 2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (4668.1g, 27.58mol) into 70kg of purified water, stir and begin to heat up to 50°C, add L-(+)- Tartaric acid (6209.2g, 41.37mol), the system was dissolved, heat-retained for 2 hours, cooled to 25°C, stirred and crystallized for 4 hours, suction filtered, dried, and then 70kg of purified water was added, the temperature was raised to 77°C under stirring, the system was dissolved, and heat-preserved for reaction 2h, cooled to 25°C, stirred and crystallized for 4h, filtered with suction, and dried; added 15.9kg of purified water, added concentrated hydrochloric acid dropwise under stirring until the system was dissolved, adjusted the pH to ≥13 with 50wt% NaOH solution, filtered with suction, and dried to obtain White solid (Intermediate I) 2250g, the yield is 48.2%, the purity shown by HPLC is 99.86%, the purity of isomer detection is:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com