Bendamustine hydrochloride preparation method suitable for industrial production
A bendamustine hydrochloride compound technology, applied in the field of preparation of bendamustine hydrochloride raw materials, can solve the problems of unqualified products, long heating and concentration time, and many dimer impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
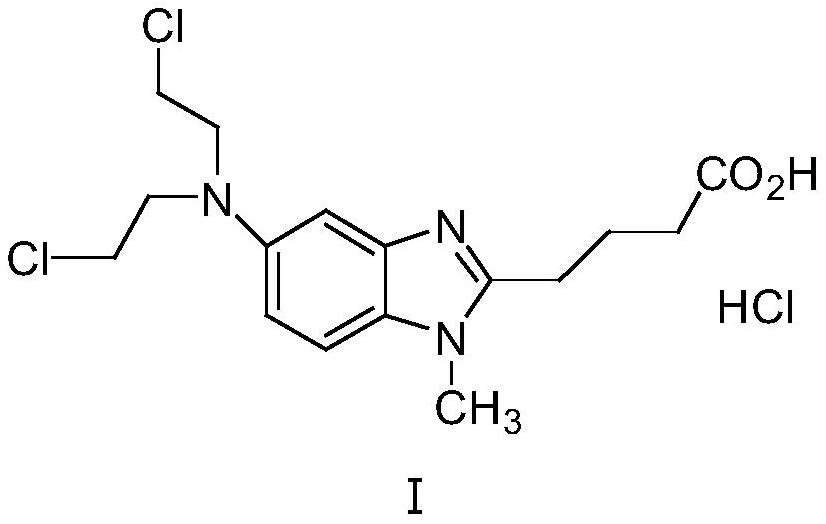
[0041] Preparation and refining (60g scale) of embodiment 1 bendamustine hydrochloride
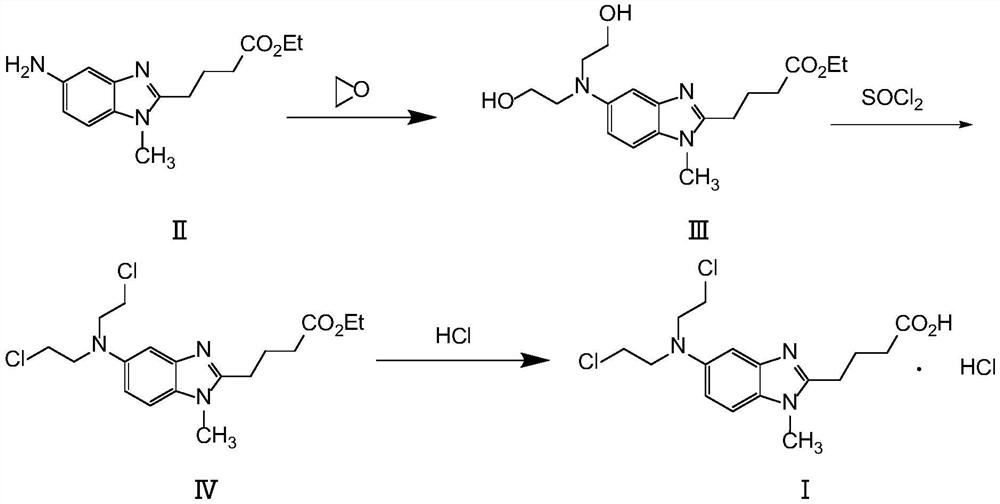
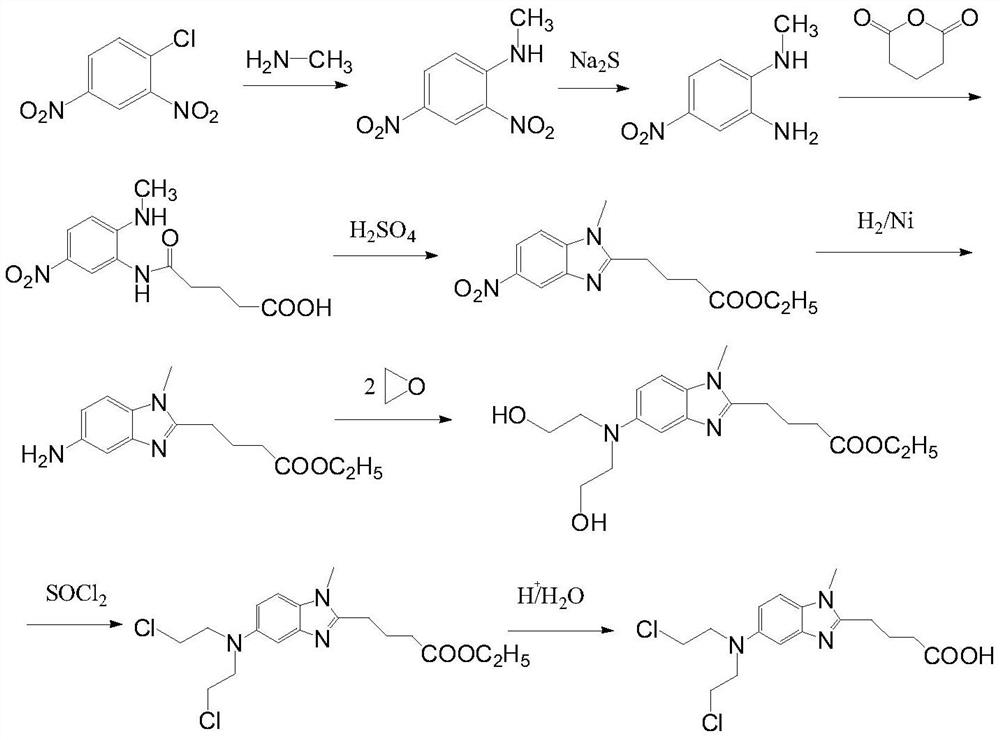
[0042] 1) Preparation of ethyl 4-{5-[bis-(2-hydroxyethyl)amino]-1-methyl-2-benzimidazole}butyrate
[0043] Stir and dissolve ethyl 5-amino-1-methyl-1H-2-benzimidazole butyrate (60g, 0.23mol), 600mL of water, and 300mL of glacial acetic acid in a 2L reaction flask, cool to -5~0°C, Add 120mL ethylene oxide, and control the temperature until the reaction is complete. The potassium carbonate solution adjusted the pH to 7.0-7.3, extracted with 300 mL×3 dichloromethane, combined the organic phases, washed with 200 mL×3 purified water, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a brown solid. Then add 1200mL ethyl acetate and heat to 70-80°C to dissolve, cool to room temperature to crystallize, filter and dry to obtain 63g of light brown solid with a purity of 99.3%. Yield 78.5%.
[0044] 2) Preparation of ethyl 4-{5-[bis-(2-chloroethyl)amino]-1-methyl-2-benzi...
Embodiment 2
[0050] The preparation of embodiment 2 bendamustine hydrochloride (60g scale)
[0051] 1) Preparation of ethyl 4-{5-[bis-(2-chloroethyl)amino]-1-methyl-2-benzimidazole}butyrate
[0052] 4-{5-[bis-(2-hydroxyethyl)amino]-1-methyl-2-benzimidazole} ethyl butyrate (60g, 0.17mol) prepared according to the method of Example 1 step (1) ) with 600mL of dichloromethane dissolved in a 2L reaction flask, cooled to -5 ~ 0°C, 30mL of thionyl chloride was added dropwise, and reacted at room temperature for 2 hours after the dropwise reaction was complete. Add 300 mL of dichloromethane to the yellow oil, stir to precipitate a solid, and dry in vacuo to obtain 61 g of a light gray solid. Yield 92%.
[0053] 2) preparation of bendamustine hydrochloride
[0054] Add 60g of the solid obtained in the previous step and 600mL of concentrated hydrochloric acid to the reaction flask equipped with tail gas absorption, and heat to reflux for 4 hours. After the reaction is completed, cool to room temp...
Embodiment 3
[0055] The preparation of embodiment 3 bendamustine hydrochloride (60g scale)
[0056] 1) Preparation of ethyl 4-{5-[bis-(2-chloroethyl)amino]-1-methyl-2-benzimidazole}butyrate
[0057] 4-{5-[bis-(2-hydroxyethyl)amino]-1-methyl-2-benzimidazole} ethyl butyrate (60g, 0.17mol) prepared according to the method of Example 1 step (1) ) with 600mL of dichloromethane dissolved in a 2L reaction flask, cooled to -5 ~ 0°C, 30mL of thionyl chloride was added dropwise, and reacted at room temperature for 2 hours after the dropwise reaction was complete. Add 360 mL of acetone to the yellow oil, stir to precipitate a solid, and dry in vacuo to obtain 59 g of a light gray solid. Yield 89.5%.
[0058] 2) preparation of bendamustine hydrochloride
[0059] In the reaction bottle equipped with tail gas absorption, add 59g of the solid obtained in the previous step, 600mL of concentrated hydrochloric acid, and heat to reflux for 4h. After the reaction is completed, cool to room temperature and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com