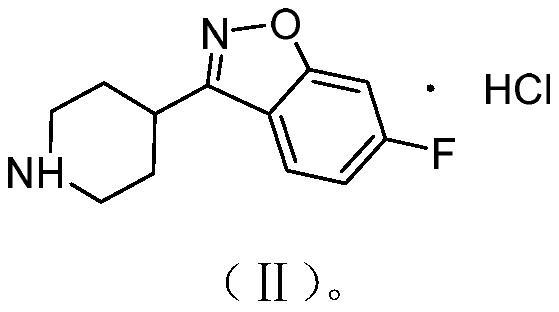
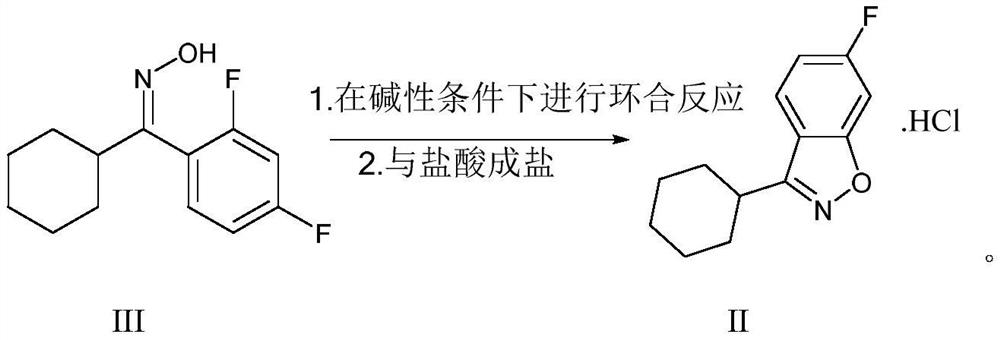
Purification method of 6-fluoro-3-(4-piperidyl)-1,2-benzoisoxazole hydrochloride
A technology of benzisoxazole hydrochloride and a purification method, applied in the field of organic chemical synthesis, can solve the problems of easy-to-encapsulate impurities, cumbersome operation, difficult to remove impurities of dimer of formula VI, etc., and achieves the effect of process stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]Preparation of 6-fluorine-3- (4-piperidinyl) -1,2-benzozole hydrochloride (preparation of the method of patented EP-196132)
[0032]In a mixture containing 65 parts of 1,3-difluorobenzene, 130 parts of aluminum chloride and 195 parts of dichloromethane, 95 parts of 1-acetyl-4-piperidine-carbonyl chloride at 65 is added dropwise with stirring. After the solution in dichloromethane, after all were dripped, stirring was continued for 3 hours at room temperature, and the reaction mixture was injected into a mixture of crushed ice and hydrochloric acid, and the product was extracted with dichloromethane. The organic layer was dried, filtered, evaporated, and 48 parts of 1-acetyl-4- (2,4-difluorobenzoyl) piperidine was 36%.
[0033]A mixture containing 48 parts of 1-acetyl-4- (2,4-difluorobenzoyl) piperidine and 180 parts of 6N hydrochloric acid solution was stirred and refluxed for 5 hours. The reaction mixture was evaporated, the residue was stirred in 2-propanol, and the product was fi...
Embodiment 2
[0038]Example 1 obtained 6-fluoro-3- (4-piperidyl) -1,2-benzoxazole hydrochloride (22 g) was added to 105 ml of anhydrous ethanol, then 40 ml of water, then heated to 70 ~ 80 ° C refluxed to dissolution, cooling to 60 ~ 70 ° C, stirring was stirred for 60 minutes, then continued to cool down to -5 ~ 5 ° C, stirring was stirred for 60 minutes, filtrate, dried, purified 6-fluoro-3- (4-piperidinyl) -1,2-benzoxazole hydrochloride purity is 99.90%, wherein the content of the formula V dimer impurities is Nd (no detection), the yield is 90.27%, and the experiment shows The technical solution can effectively remove the V dimer impurities and the yield is high.
Embodiment 3
[0040]150 ml of toluene, 150 ml of drinking water, 45 g of solid toline hydroxide powder and 35 g of soda oxime wet material were added at 80 ° C for 6 hours at 80 ° C for 6 hours, and the material was allowed to stand at room temperature to stand in the layer. The lower aqueous layer was extracted with toluene, combined with toluene phase, distillation under reduced pressure. After evaporation, 175 ml of anhydrous ethanol dissolved material was added, and 10 to 13 ml of hydrochloric acid was added thereto, and the pH = 2.0 ~ 3.0 was added thereto, and 67 ml of drinking water was added, and the heated to 80 ° C to reflux, and then down to 70 ~ 80. At ° C, stirring was stirred for 1 hour, continued to cool down to 20 to 40 ° C, then continued to cool down to -5 ~ 0 ° C, stirred for 1 h, stirred, filtered, drunk with 50 ml of anhydrous ethanol, dried. White powder solid 6-fluoro-3- (4-piperidyl) -1,2-benzo isoxazole hydrochloride salt, pure product content is 99.90%, yield 85.27%, whe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com