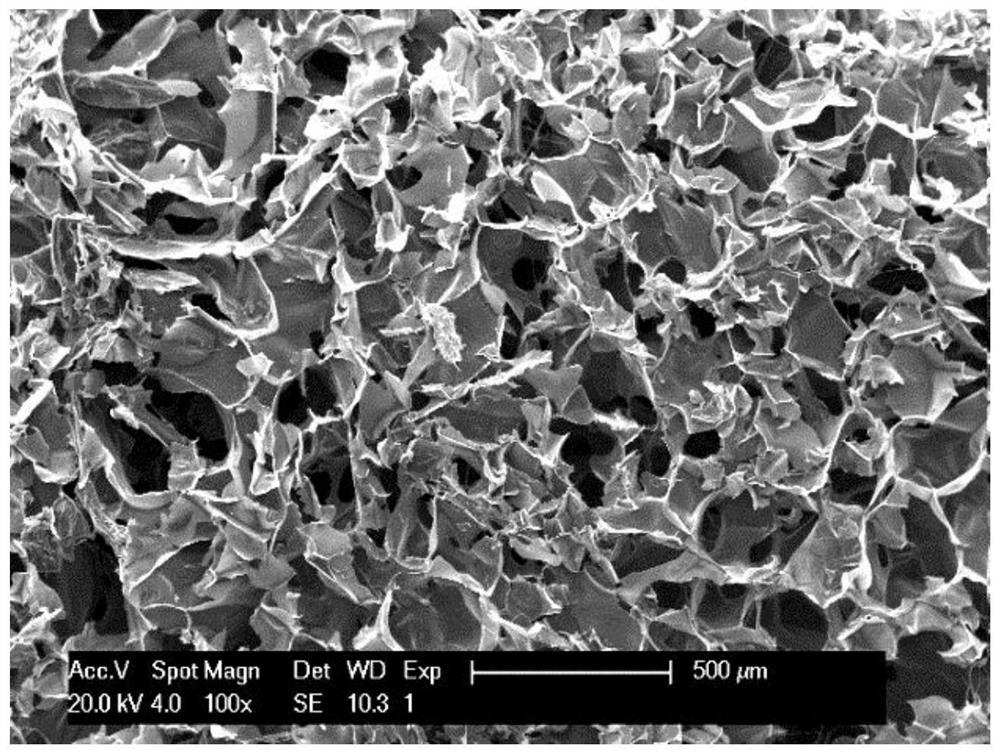
Tannic acid cross-linked chitosan/gelatin liquid-absorbing hemostatic antibacterial sponge and preparation method thereof
A cross-linked chitosan, hemostasis and antibacterial technology, applied in the directions of pharmaceutical formulation, application, medical science, etc., can solve the problems of inability to provide the mechanical properties of hemostatic dressings, poor biocompatibility of acetalized sponges, and unfavorable wound healing, etc. Conducive to large-scale production, good market application prospects, and cheap market prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A tannic acid cross-linked chitosan / gelatin liquid-absorbing hemostatic antibacterial sponge and a preparation method thereof, specifically comprising the following steps:
[0044] S1, after dissolving 3g of chitosan with 22% acetic acid with a mass fraction, magnetically stir for 2h, and centrifuge to collect the supernatant; adjust the pH value of the collected supernatant to 9 with 0.5M NaOH, and place it statically to make the chitosan Completely precipitated, freeze-dried for 12 hours, obtained chitosan after purification, and set aside;
[0045] S2. Dissolve 8g of gelatin in acetic acid with a mass fraction of 20%, stir slowly, and heat to 60±0.5°C. After the gelatin is completely dissolved, add 2g of EDCI. After stirring for 15min, add chitosan and 2g of purified chitosan in step S1 at a time. DMAP, continuously stirred and heated for 6 hours to obtain a reaction solution;
[0046] S3. Dialyze the reaction solution obtained in step S2 with a semi-dialysis bag of...
Embodiment 2
[0052] A tannic acid cross-linked chitosan / gelatin liquid-absorbing hemostatic antibacterial sponge and a preparation method thereof, specifically comprising the following steps:
[0053] S1, after dissolving 3g of chitosan with 22% acetic acid with a mass fraction, magnetically stir for 2h, and centrifuge to collect the supernatant; adjust the pH value of the collected supernatant to 9 with 0.5M NaOH, and place it statically to make the chitosan Completely precipitated, freeze-dried for 12 hours, obtained chitosan after purification, and set aside;
[0054] S2. Dissolve 8g of gelatin in acetic acid with a mass fraction of 20%, stir slowly, and heat to 60±0.5°C. After the gelatin is completely dissolved, add 2g of EDCI. After stirring for 15min, add chitosan and 2g of purified chitosan in step S1 at a time. DMAP, continuously stirred and heated for 6 hours to obtain a reaction solution;
[0055] S3. Dialyze the reaction solution obtained in step S2 with a semi-dialysis bag of...
Embodiment 3
[0061] A tannic acid cross-linked chitosan / gelatin liquid-absorbing hemostatic antibacterial sponge and a preparation method thereof, specifically comprising the following steps:
[0062] S1, after dissolving 3g of chitosan with 22% acetic acid with a mass fraction, magnetically stir for 2h, and centrifuge to collect the supernatant; adjust the pH value of the collected supernatant to 9 with 0.5M NaOH, and place it statically to make the chitosan Completely precipitated, freeze-dried for 12 hours, obtained chitosan after purification, and set aside;
[0063] S2. Dissolve 8g of gelatin in acetic acid with a mass fraction of 20%, stir slowly, and heat to 60±0.5°C. After the gelatin is completely dissolved, add 2g of EDCI. After stirring for 15min, add chitosan and 2g of purified chitosan in step S1 at a time. DMAP, continuously stirred and heated for 6 hours to obtain a reaction solution;
[0064] S3. Dialyze the reaction solution obtained in step S2 with a semi-dialysis bag of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com