Application of polypeptide AT03 in medicine used for treating primary biliary cholangitis
An AT03, primary technology, applied in the field of biochemistry, can solve problems such as unsatisfactory treatment of PBC, achieve the effects of good biological activity, low cost, and easy to scale up production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of polypeptide AT03
[0031] The amino acid sequence of polypeptide AT03 is: Val-His-Val-Val-R 1 ; where R 1 =-NH 2 . In this example, AT03 was synthesized by the Fmoc solid-phase polypeptide synthesis method from the carboxy-terminus to the amino-terminus, and the amino acids of the polypeptide AT03 were connected sequentially according to the sequence of amino acids mentioned above.
[0032] (1) Polypeptide synthesis
[0033] The preparation condition of the present invention adopts MBHAR (Amide-MBHA-Resin amide-protected MBHA resin) or Wang resin, and synthesizes by removing the Fmoc protecting group with the method of activating lipid.
[0034] The indene detection reagent of the present invention comprises following components:
[0035] Reagent 1: 20g phenol / 5mL ethanol;
[0036] Reagent 2: 0.05 0.001M KCN (water) / 2.5mL pyridine;
[0037] Reagent 3: 0.5g ninhydrin / 10mL ethanol;
[0038] The usage ratio of the indene detection...
Embodiment 2
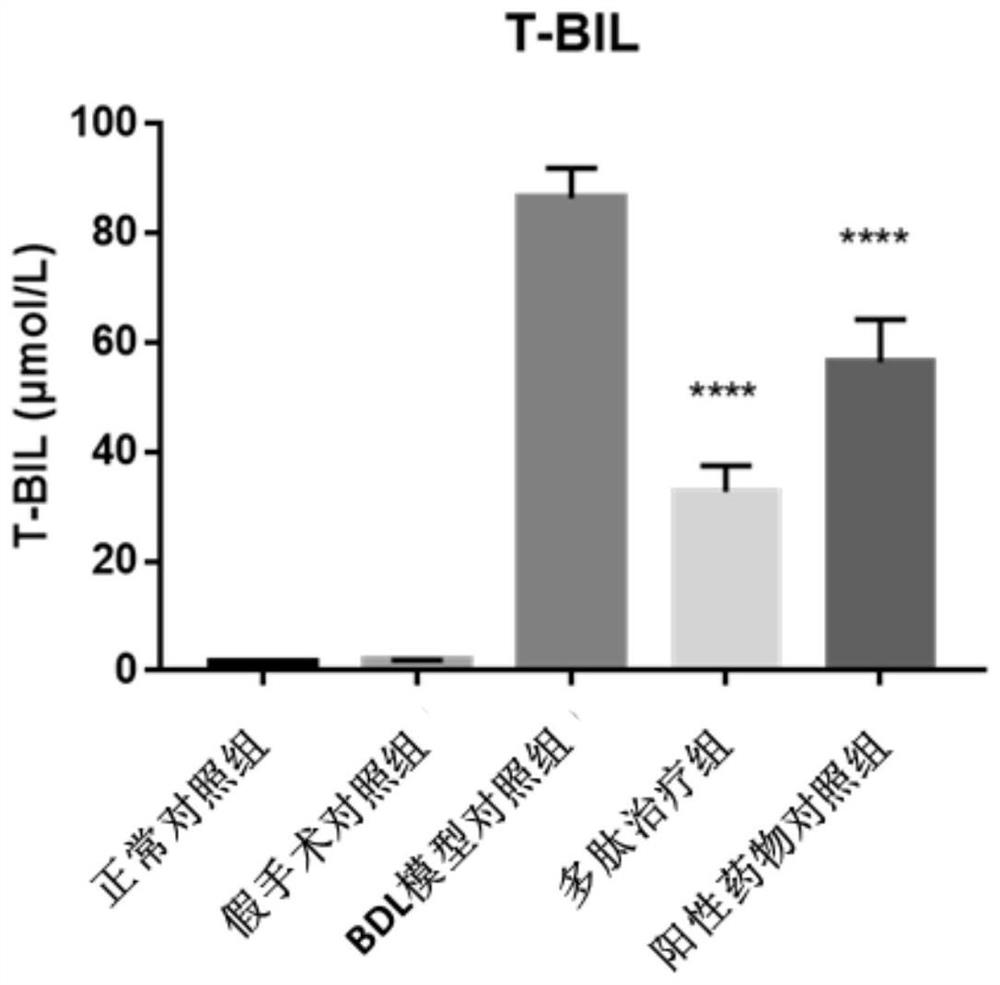
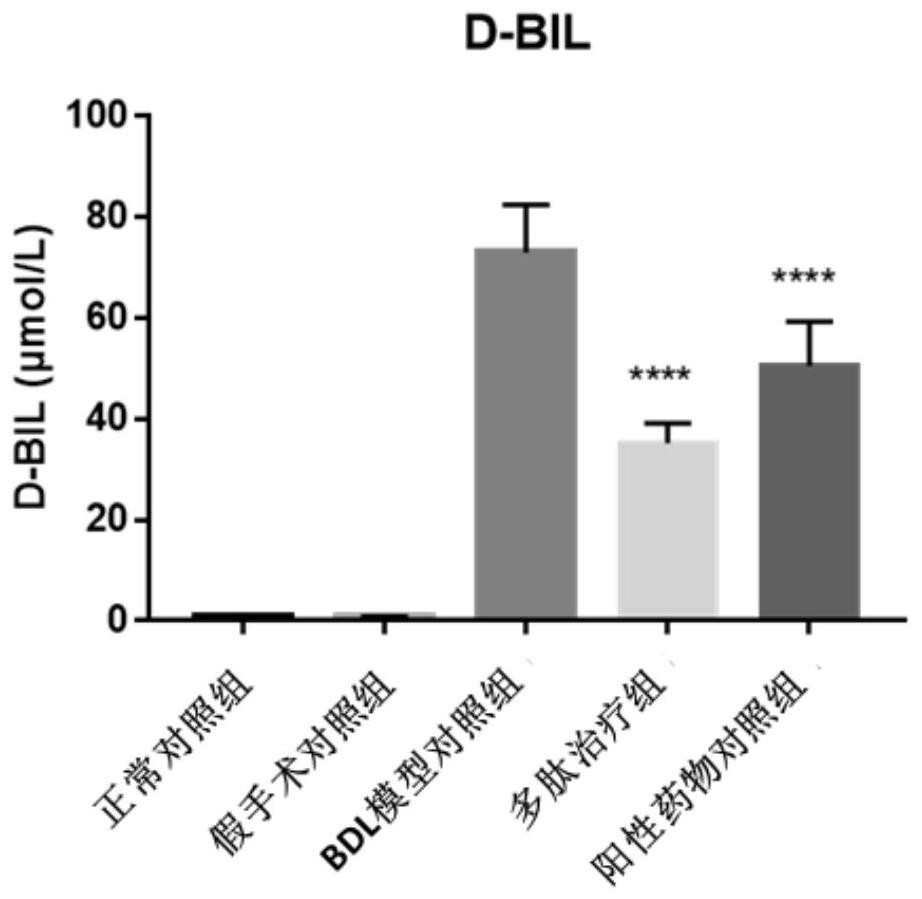
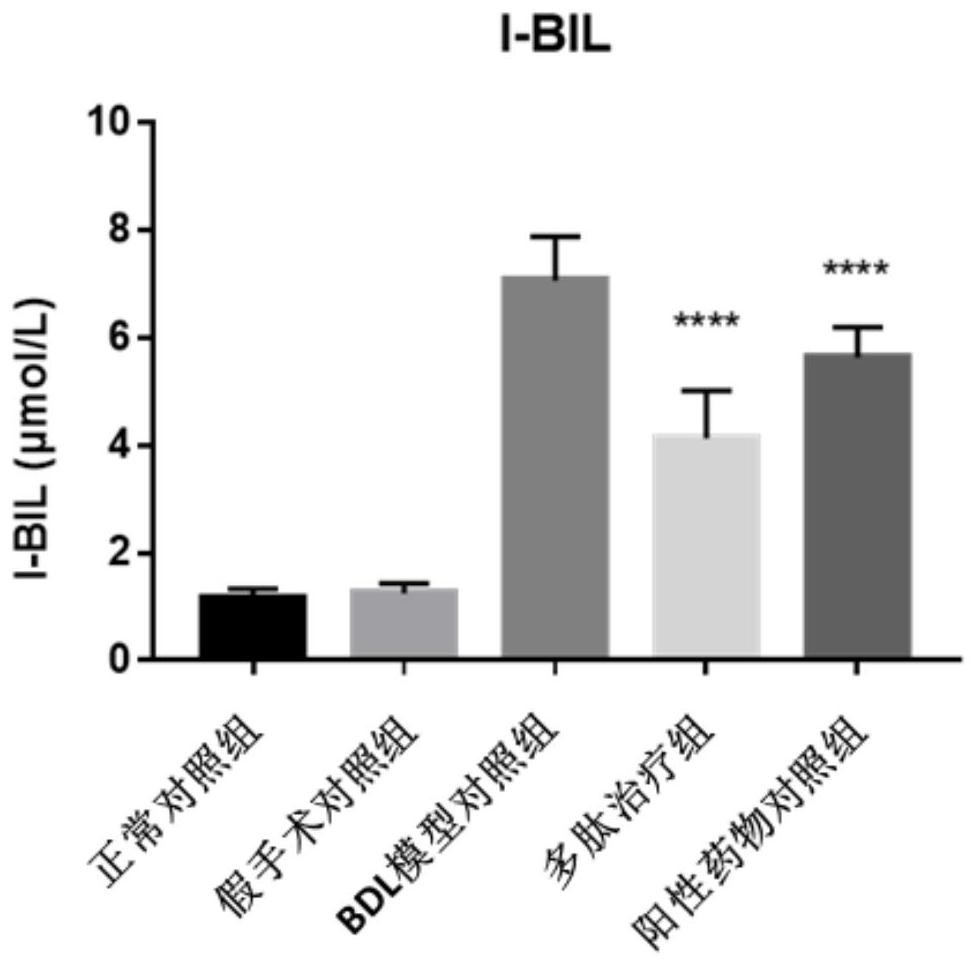
[0057] Example 2, the improved therapeutic effect of the polypeptide AT03 of the present invention on the common bile duct ligation (BDL) rat model of cholestatic liver cirrhosis
[0058] The specific method of this embodiment is as follows.
[0059] 1. Test drug: polypeptide AT03, positive control drug (ursodeoxycholic acid, UDCA). Storage conditions -20°C.
[0060] Modeling method: before the operation, the experimental animals were anesthetized with 3% pentobarbital sodium at a dose of 60 mg / kg. After routine disinfection of the abdominal skin, an incision was made along the midline of the abdomen to expose the common bile duct. After side suturing and ligation, no active bleeding in the abdominal cavity was detected, the abdomen was routinely closed. In the sham operation group, the abdomen was only opened and the common bile duct was freed, and antibiotics were given for postoperative care according to the animal reaction. Animals in the normal control group were not giv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com