Resin composition, composite material and preparation method thereof
A resin composition and composition technology, applied in the field of resin composition, composite material and its preparation, can solve the problems of cumbersome operation process, poor process adaptability, low safety factor, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
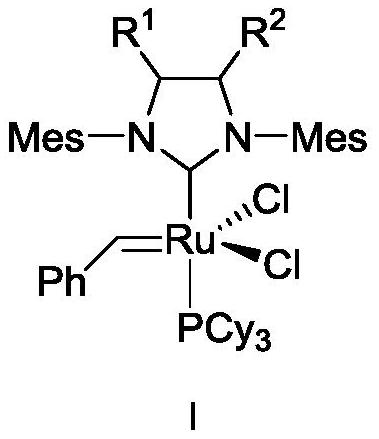
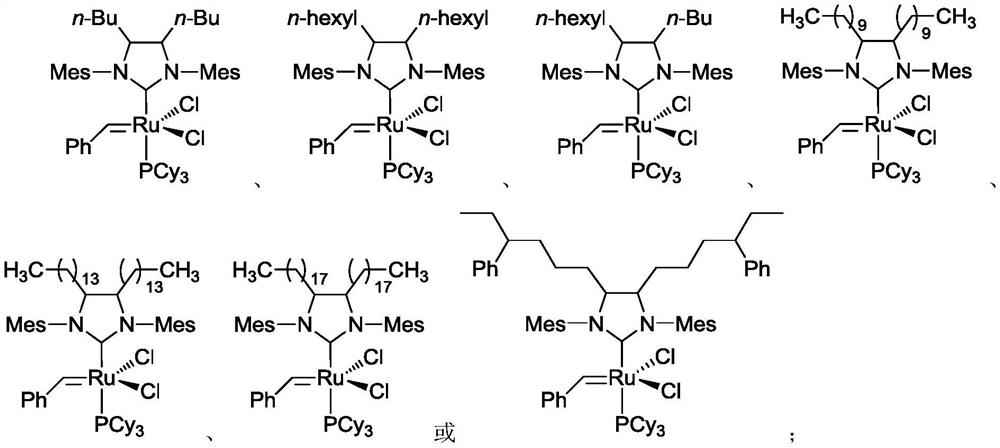
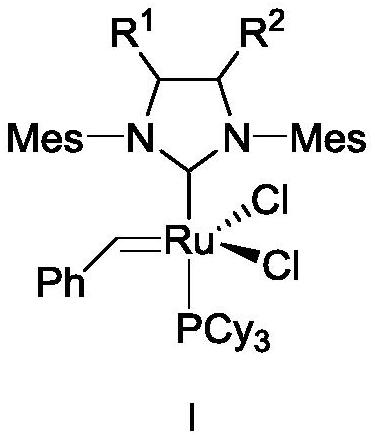
[0153] Embodiment 1: Catalyst 1,3-bis(2,4,6-trimethylphenyl)-2-(4,5-dibutylimidazolidinylidene) (dichlorobenzylidene) containing butyl substituent base) (tricyclohexylphosphine) ruthenium (I-1) synthesis
[0154] The synthesis of butyl substituent catalyst I-1 comprises the following steps:
[0155] 1) Preparation of N,N'-bis(2,4,6-trimethyl)phenylethylenediimine (9)
[0156]
[0157] Add 3.73 mL of glyoxal (8) aqueous solution (40%) and 80 mL of methanol successively into a 500 mL three-neck flask equipped with a dropping funnel, and stir to dissolve the glyoxal. Add 9.12 mL of m-trimethylaniline (7) and 10 mL of methanol to the dropping funnel, and slowly drop them into the flask. The temperature of the reaction solution was controlled at about 22° C., and stirred for 12 hours. During this process, a bright yellow precipitate slowly precipitated from the reaction solution. After the reaction, the reaction liquid was filtered to obtain a yellow solid, which was washed ...
Embodiment 2
[0167] Embodiment 2: Containing hexyl substituent catalyst 1,3-bis(2,4,6-trimethylphenyl)-2-(4,5-dihexyl imidazolidinylidene) (dichlorobenzylidene) Synthesis of (tricyclohexylphosphine)ruthenium(I-2)
[0168] Containing the synthesis of hexyl substituent catalyst 1-2 comprises the following steps:
[0169] 1) Preparation of N,N'-bis(2,4,6-trimethyl)phenylethylenediimine (9)
[0170]
[0171] Add 3.73 mL of glyoxal (8) aqueous solution (40%) and 80 mL of methanol successively into a 500 mL three-neck flask equipped with a dropping funnel, and stir to dissolve the glyoxal. Add 9.12 mL of m-trimethylaniline (7) and 10 mL of methanol to the dropping funnel, and slowly drop them into the flask. The temperature of the reaction solution was controlled at about 22° C., and stirred for 12 hours. During this process, a bright yellow precipitate slowly precipitated from the reaction solution. After the reaction, the reaction liquid was filtered to obtain a yellow solid, which was ...
Embodiment 3
[0181] Example 3: 4-butyl-5-hexyl-1,3-bis(2,4,6-trimethylphenyl)-2-(imidazolidinylidene)(benzylidene)(tricyclohexyl Preparation of phosphine) ruthenium(I-3) dichloride
[0182] 1.1) Preparation of N,N'-bis(2,4,6trimethylphenyl)dodecane-5,6-diamine (14)
[0183]
[0184] Under nitrogen protection, add 2.92g N,N'-bis(2,4,6-trimethyl)phenylethylenediimine (9) (Mw: 292.46g / mol; 10.0 mmol), 100mL tetrahydrofuran was stirred to dissolve it. Then, the ampoule was placed in an ethanol cold bath at -78°C, stirred and cooled. After the reaction solution was fully cooled, 4.17 mL (10.0 mmol) of hexyllithium (2.4 M solution in toluene) was slowly added dropwise with a syringe. The reaction solution was cooled to -78°C again, and 6.25 mL (10.0 mmol) of butyllithium (1.6 M hexane solution) solution was slowly added dropwise with a syringe. After the addition was complete, the reaction mixture was slowly raised to room temperature with stirring, and continued to stir for 0.5 h. Then,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com