Large-scale preparation technology for high-purity mesalazine enteric-coated sustained-release tablet preparations
A technology of mesalazine and preparation technology, which is applied in the field of preparation of chemical drugs, and can solve problems such as physical health damage and ineffective improvement of treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
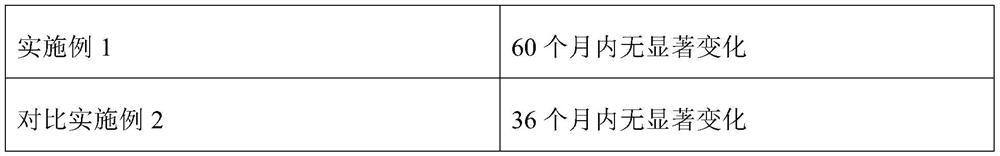
Embodiment 1
[0023] In parts by weight, dissolve 100 parts of polyglutamic acid in 300 parts of water, add N-hydroxysuccinimide NHS solution and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide The hydrochloride EDC solution (volume ratio of 1:1) was reacted at room temperature for 6 h to obtain an activated polyglutamic acid solution. Then the activated polyamino acid solution was added with a mass ratio of 1:0.3 ethylene glycol dimethacrylate and azobisisobutyronitrile, mixed uniformly, and reacted at 3 °C for 0.5 h, and then the product was mixed with 300 parts of polyethylene The aqueous alcohol solution (mass fraction of 25 wt %) was mixed, and the reaction was stirred at 30° C. for 2 h to obtain a polyvinyl alcohol-polyglutamic acid copolymer. Under stirring, slowly add 50 parts of mesalazine and 200 parts of polyvinyl alcohol-polyglutamic acid copolymer into the rapid wet mixing granulator, add 15 parts of sodium alginate immediately after adding, keep stirring, until A suitable soft ...
Embodiment 2
[0025] Dissolve 100 parts of polyglutamic acid in 300 parts of water, add N-hydroxysuccinimide NHS solution and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride EDC solution ( The volume ratio is 1:2), and the reaction is carried out at room temperature for 4 h to obtain an activated polyglutamic acid solution. Then add ethylene glycol dimethacrylate and azobisisobutyronitrile in a mass ratio of 1:0.3 to the activated polyamino acid solution, mix them evenly, and react at 0 °C for 1 h, and then mix the product with 400 parts of polyvinyl alcohol. The aqueous solution (mass fraction of 30 wt %) was mixed, and the reaction was stirred at 40° C. for 3 h to obtain a polyvinyl alcohol-polyglutamic acid copolymer. Under stirring, slowly add 50 parts of mesalazine and 250 parts of polyvinyl alcohol-polyglutamic acid copolymer into the rapid wet mixing granulator, and immediately add 5 parts of hydroxypropyl methylcellulose after adding, Keep stirring until a suitable soft...
Embodiment 3
[0027]Dissolve 100 parts of polyglutamic acid in 200 parts of water, add N-hydroxysuccinimide NHS solution and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride EDC solution ( The volume ratio is 1:2), and the reaction is carried out at room temperature for 8 hours to obtain an activated polyglutamic acid solution. Then add ethylene glycol dimethacrylate and azobisisobutyronitrile in a mass ratio of 1:0.5 to the activated polyamino acid solution, mix them evenly, and react at 5°C for 0.5h, and then mix the product with 400 parts of polylactic acid. The aqueous solution (mass fraction of 20 wt %) was mixed, and the reaction was stirred at 35° C. for 2 h to obtain a polylactic acid-polyglutamic acid copolymer. Under stirring, slowly add 50 parts of mesalazine and 400 parts of polylactic acid-polyglutamic acid copolymer into the rapid wet mixing granulator, add 20 parts of agar immediately after adding, and keep stirring until a suitable Soft material; after the soft m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com