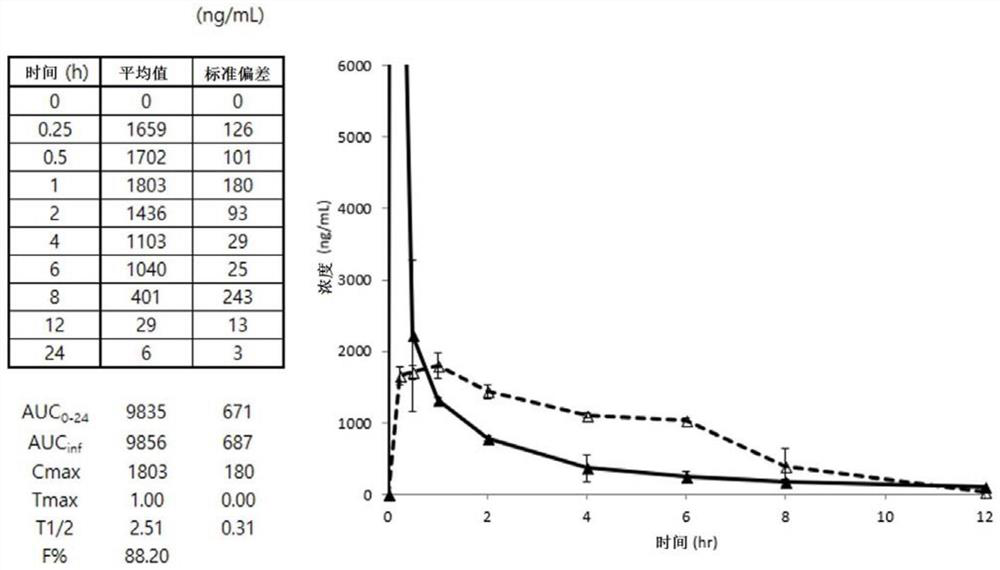
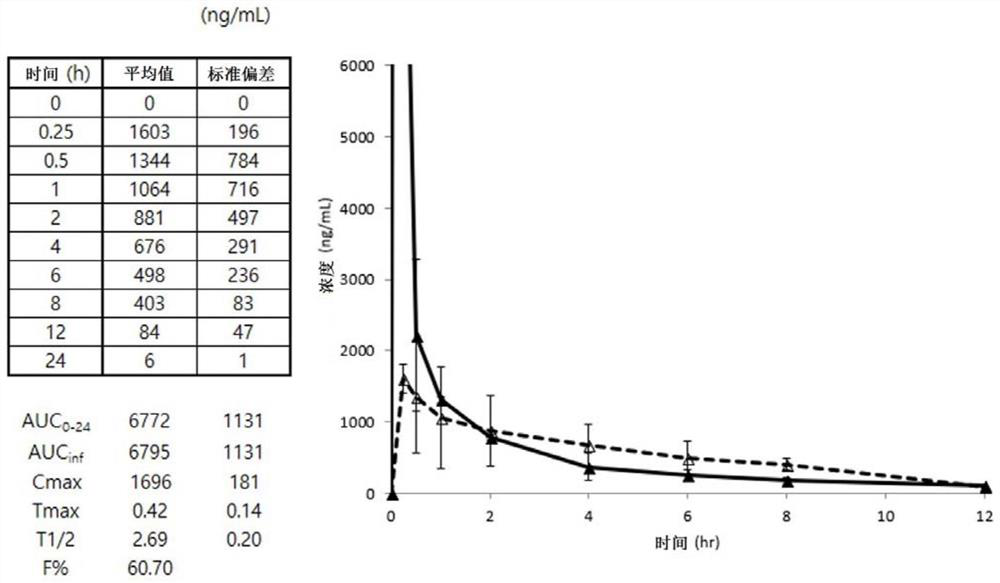
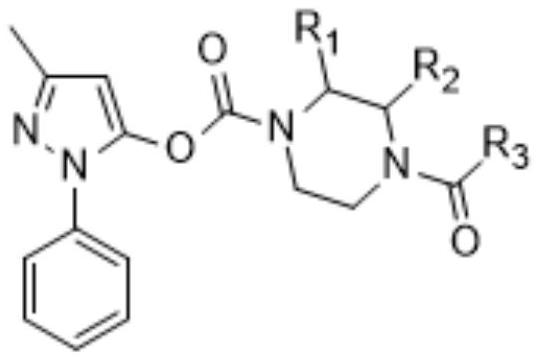
Edaravone prodrug compound and pharmaceutical use thereof in treatment or alleviation of neurodegenerative or motor neuron disease
A compound and drug technology, applied in the field of edaravone prodrug compound and its medical use in the treatment or improvement of neurodegenerative or motor neuron diseases, can solve the problem of insufficient improvement of the bioavailability of edaravone , Undisclosed or implied Edaravone plasma concentration, undisclosed pharmacokinetics and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0058] Reference Example 1: 3-methyl-1-phenyl-1H-pyrazol-5-yl 4-methylpiperazine-1-carboxylate hydrochloride
[0059]
[0060] 1.0 g of 1-methylpiperazine was dissolved in 10 ml of dichloromethane, and 1.2 ml of pyridine (1.5 eq.) was added. The reaction solution was cooled to below 0° C. under an argon atmosphere, and then 3.5 g (1.2 eq.) of triphosgene diluted in 15 ml of dichloromethane was slowly added thereto. After stirring at room temperature for 2 hours, it was washed with 25 ml of saturated brine, and the organic layer was separated. After drying over anhydrous magnesium sulfate, it was concentrated under reduced pressure to obtain a yellow oil. After complete dissolution by adding 10 ml of acetonitrile, 1.74 g (1.0 eq.) of edaravone and 9.76 g (3 eq.) of cesium carbonate were added thereto. After stirring at room temperature for 4 hours, the reaction solution was filtered using celite, and the filtrate was recovered and concentrated under reduced pressure. The ...
preparation example 1
[0063]
[0064] After dissolving the benzyl piperazine-1-carboxylated derivative in 10 times the volume of methylene chloride, 1.2 equivalents of triethylamine and 1.1 equivalents of the active ester compound were added. The mixture was stirred at room temperature under an argon atmosphere for 2 hours, washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was dissolved in tetrahydrofuran, 5% of palladium adsorbed on carbon of 10 wt% purity was added thereto, and then stirred at room temperature under normal pressure of hydrogen. After the reaction is completed, the reaction solution is filtered, and the filtrate is recovered and concentrated under reduced pressure. Purification by silica gel column chromatography (eluent: a mixed solution of dichloromethane and methanol) afforded an acylated piperazine intermediate. 10 times the volume of dichloromethane was added thereto to dissolve, and 1.5 equivalents o...
preparation example 2
[0066]
[0067] Dissolve benzyl piperazine-1-carboxylate derivative in 5 times volume of dichloromethane and 5 times volume of N-methyl-2-pyrrolidone (N-methyl-2 -pyrrolidone, NMP), then add 1.0 equivalent of amino acid whose amino group is protected by t-butoxycarbonyl (Boc), 1.1 equivalent of diisopropylcarbodiimide (diisopropylcarbodiimide, DIPC) and 1.2 equivalent of three Ethylamine. The mixture was stirred at room temperature under an argon atmosphere for 2 hours, washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was dissolved in tetrahydrofuran, 5% palladium adsorbed on 10 wt% pure carbon, and stirred at room temperature under atmospheric pressure of hydrogen. After the reaction was completed, the reaction solution was filtered, and the filtrate was recovered and concentrated under reduced pressure. Purification by silica gel column chromatography (eluent: a mixed solution of dichloromethane an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com