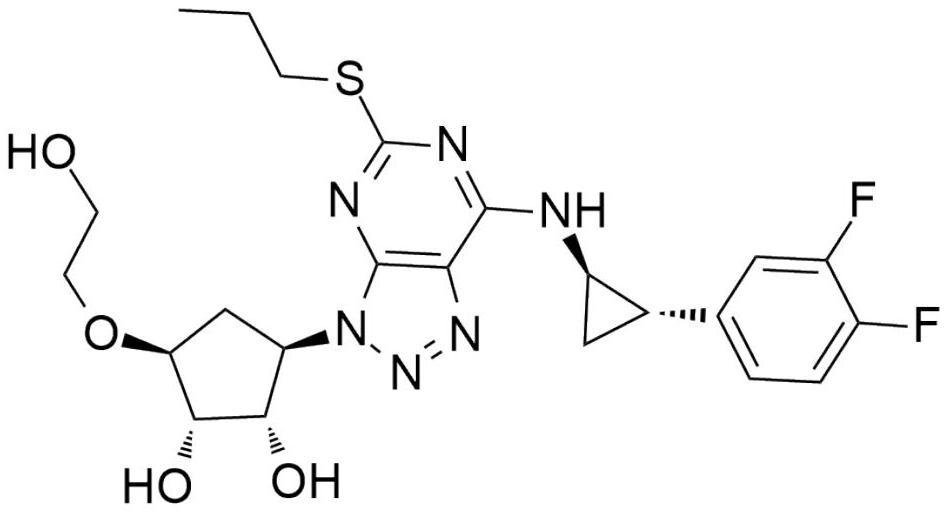
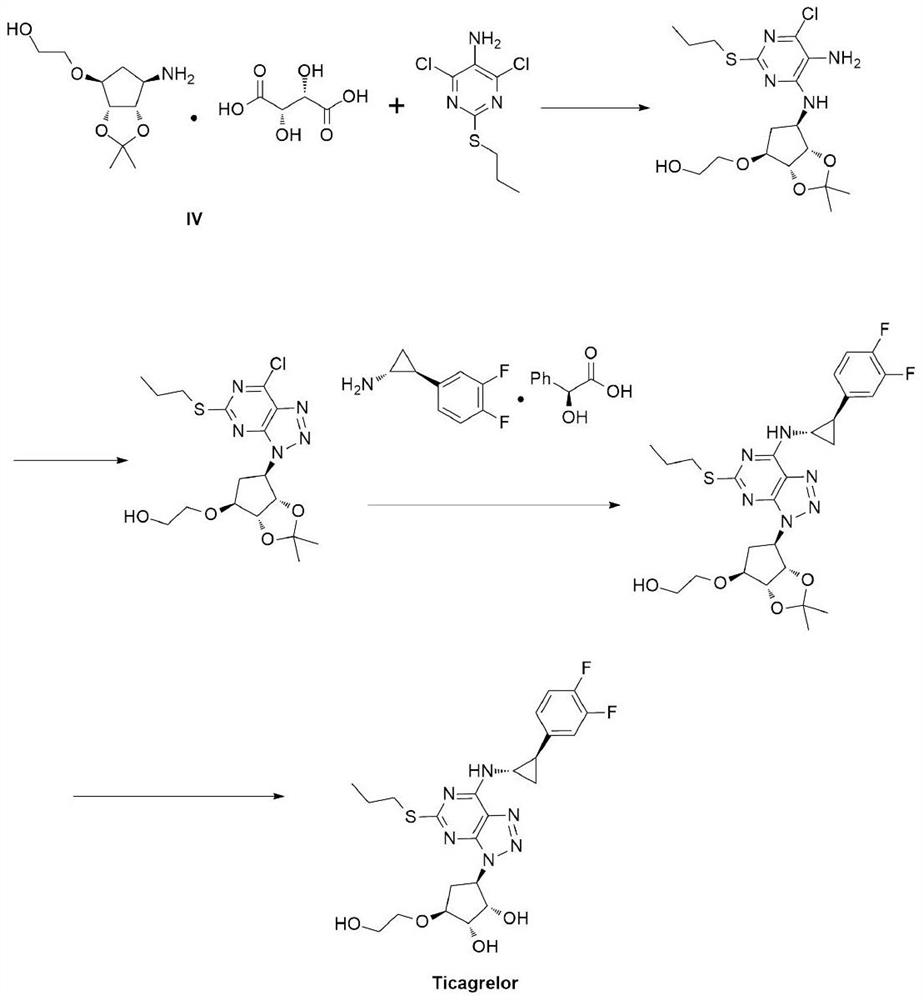
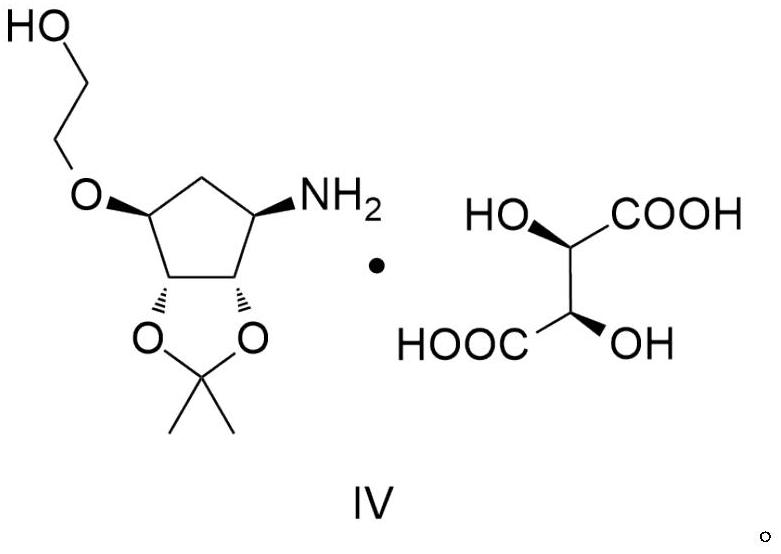
A kind of synthetic method of ticagrelor key intermediate
A technology of ticagrelor and synthetic method, which is applied in the field of medicine, can solve the problems of undiscovered intermediate synthesis information, etc., and achieve the effects of increasing yield, simplifying process and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment one: the preparation of compound III
[0036] Put 100g of compound I and 800mL of tetrahydrofuran into a 2L four-necked bottle 1, cool down to 0-10°C, slowly add 40.1g of potassium tert-butoxide in batches to bottle 1, continue stirring for 15 minutes after the addition, and then control the temperature for 0-10°C. Slowly add 62.5g of sodium bromoacetate in batches to bottle 1 at ℃, continue to stir for 2h after the addition, and then raise the temperature to 10-20℃ and slowly add 45.7g of ethyl chloroformate into bottle 1 dropwise. The temperature was controlled at 10-20°C and the reaction was stirred for 1 hour, then the temperature was lowered to 0-10°C and 29.7 g of sodium borohydride was slowly added in batches. After incubating at 0-10°C for 4 hours, add 30% acetic acid aqueous solution dropwise to quench the reaction, adjust the pH to 4-5, concentrate under reduced pressure to remove tetrahydrofuran, then add 500 mL of water and extract with ethyl acet...
Embodiment 2
[0038] Embodiment two: the preparation of compound III
[0039] Put 100g of compound I and 800mL tetrahydrofuran into a 2L four-necked bottle 1, cool down to 0-10°C, slowly add 68.8g of sodium tert-butoxide into bottle 1 in batches, continue stirring for 15 minutes after the addition, and then control the temperature for 0-10°C. Slowly add 49.5g of bromoacetic acid in batches to bottle 1 at ℃, continue to stir for 2h after the addition, then raise the temperature to 10-20℃ and slowly add 42.2g of ethyl chloroformate dropwise into bottle 1. Control the temperature at 10-20°C and stir the reaction for 1h, then lower the temperature to 0-10°C and slowly add 30.9g of sodium borohydride in batches. After incubating at 0-10°C for 4 hours, add 30% acetic acid aqueous solution dropwise to quench the reaction, adjust the pH to 4-5, concentrate under reduced pressure to remove tetrahydrofuran, then add 500 mL of water and extract with ethyl acetate (200 mL×3), and combine the organic ph...
Embodiment 3
[0041] Embodiment three: the preparation of compound III
[0042] Put 100g of compound I and 800mL tetrahydrofuran into a 2L four-necked bottle 1, cool down to 0-5°C, slowly add 7.8g of sodium hydride in batches to bottle 1, continue stirring for 0.5h after the addition, and then control the temperature at 0-10°C to Slowly add 57.3g of sodium bromoacetate in batches to bottle 1, continue to stir for 2 hours after the addition, then raise the temperature to 10-20°C and slowly add 38.7g of ethyl chloroformate dropwise to bottle 1. The temperature was controlled at 10-20°C and the reaction was stirred for 1 hour, then the temperature was lowered to 0-10°C and 24.8 g of sodium borohydride was slowly added in batches. After incubating at 0-10°C for 4 hours, add 30% acetic acid aqueous solution dropwise to quench the reaction, adjust the pH to 4-5, concentrate under reduced pressure to remove tetrahydrofuran, then add 500 mL of water and extract with ethyl acetate (200 mL×3), and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com