Protamine oligopeptide modified paclitaxel liposome and preparation method thereof
A protamine and paclitaxel technology, which is applied in the directions of liposome delivery, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problem that biological macromolecules are difficult to cure, affect drug efficacy and targeting, and have poor applicability and other problems, to achieve the effect of increasing water solubility, stable product properties, and enhancing drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
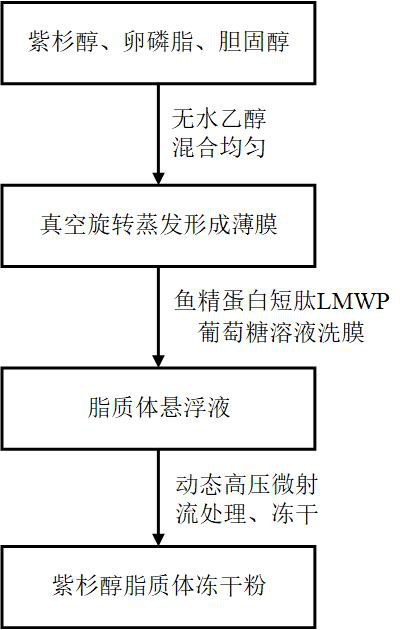
[0023] The present invention also provides a preparation method of the above paclitaxel liposome, comprising the following steps: dissolving paclitaxel, lecithin, and cholesterol in a solvent, removing the solvent after sterilization to form a film; using glucose containing protamine short peptide LMWP The membrane was washed with the solution, homogenized to obtain liposome suspension, and freeze-dried to obtain paclitaxel liposome freeze-dried powder.
[0024] The present invention has no special limitation on the type of solvent, and any type of solvent commonly used in the preparation of paclitaxel liposomes in the art can be used. In a specific embodiment of the present invention, the solvent is preferably absolute ethanol. In the present invention, the amount of absolute ethanol is calculated as lecithin, preferably 25-50mL / g lecithin, more preferably 30-45mL / g lecithin, even more preferably 35-40mL / g lecithin.
[0025] The present invention has no special limitation on ...
Embodiment 1
[0032] Accurately weigh 0.50g of paclitaxel, 15g of lecithin, 2.5g of cholesterol, dissolve in 375mL of absolute ethanol, fully stir and dissolve, after sterilizing and filtering through a 0.22μm filter element, remove the absolute ethanol by rotary evaporation to form a thin film; slowly inject 400mL of Wash the membrane with 5% protamine short peptide LMWP glucose solution, disperse for 2 minutes, and then perform homogenization treatment with dynamic high-pressure micro-jet (120MPa, 3 times) to obtain a liposome suspension; freeze the liposome suspension at -80°C Dry for 48 hours to obtain paclitaxel liposome lyophilized powder.
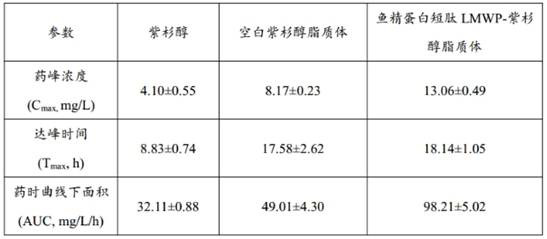
[0033] The particle size of the paclitaxel liposomes in the liposome suspension was measured by a potential-particle size analyzer as 124.43 nm, a dispersion coefficient of 0.8, and a zeta potential of -40.6mV. Using high performance liquid chromatography (chromatographic conditions: C 18 , water-acetonitrile 33:67 as mobile phase, flow rate 1.0m...
Embodiment 2
[0035] Accurately weigh 1.5g of paclitaxel, 30g of lecithin, 7.5g of cholesterol, dissolve in 750mL of absolute ethanol, fully stir to dissolve, after sterilizing and filtering through a 0.22μm filter element, remove the absolute ethanol by rotary evaporation to form a thin film; slowly inject 500mL of 2 Wash the membrane with a glucose solution of % protamine short peptide LMWP, disperse for 3 minutes, and then perform homogenization treatment with dynamic high-pressure micro-jet (200MPa, 5 times) to obtain a liposome suspension; freeze-dry the liposome suspension at -80°C 48h to obtain paclitaxel liposome lyophilized powder.
[0036]Using the same assay method as in Example 1, the encapsulation efficiency of paclitaxel liposomes was 92%, the average particle size was 121.17 nm, the dispersion coefficient was 0.85, and the zeta potential was -38.4mV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com