Method for preparing ceftibuten mother nucleus 7-ANCE
A technology of 7-ANCE and ceftibuten core, which is applied in organic chemistry and other fields, can solve the problems of physical injury of workshop workers, long steps, and increased cost of 7-ANCE raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Compound A was prepared as follows:
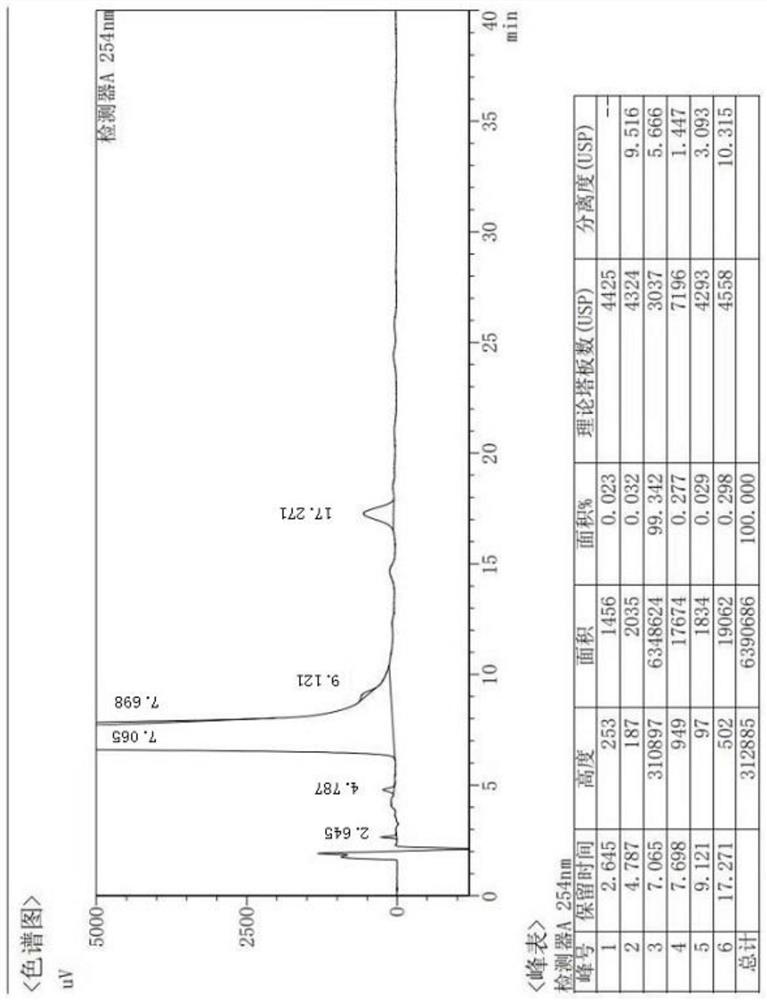
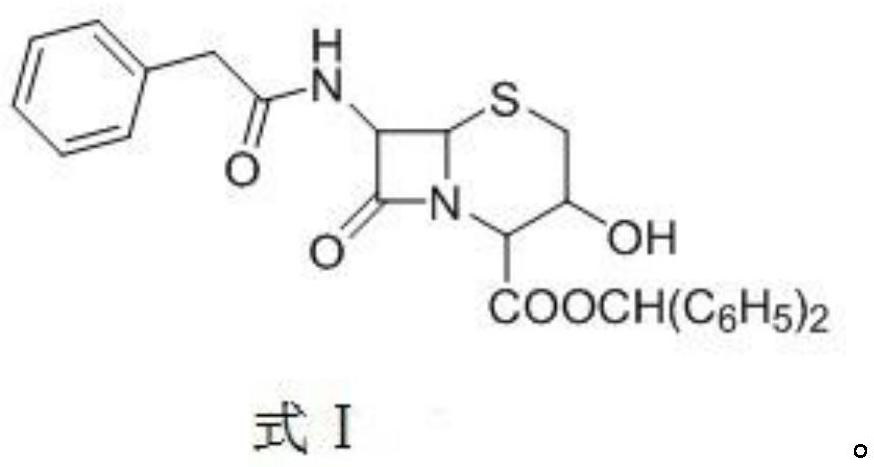
[0038] Add 50.02g (0.1mol) of 3-hydroxycephalosporin and 600mL of dichloromethane into a 1000mL reaction flask, stir for 20min, and cool down to -60~-50°C; add 11.4g (0.22mol) of potassium borohydride, and control the temperature at -60°C 200ml of methanol was added dropwise at ~-50°C, and the reaction was stirred for 2 hours. After the reaction is completed and the temperature rises to room temperature, wash with 200ml of 5% sodium chloride solution and 200ml of water successively, separate layers, concentrate and dry the organic layer under reduced pressure, and then add dichloromethane to the system: cyclohexane volume ratio = 2 : 200ml of mixed solvent crystallization of 1, filter after the temperature drops to -10~0°C, and dry under reduced pressure at 50°C to obtain compound A shown in formula 1, 45.3g of white solid, yield 90.6%, purity 98.5%, reaction The formula is as follows:
[0039]
[0040] Compound A prepared by t...
Embodiment 2
[0044] Step S1: Add 50.2g (0.1mol) of compound A and 500mL of dichloromethane into a 1000mL reaction flask, stir for 20min, and cool down to -20°C; add 58.3g (0.28mol) of phosphorus pentachloride, stir for 10 minutes and drop Add 22.1 g (0.28 mol) of pyridine, and control the temperature at -20°C to keep stirring and react for 3 hours.
[0045] Step S2: After the reaction, control the temperature below 0°C and add HCl isobutanol solution (300ml, acid mass concentration 15%) dropwise, control the temperature -5°C, keep stirring for 4 hours, filter after the stirring, and filter out the ammonium salt.
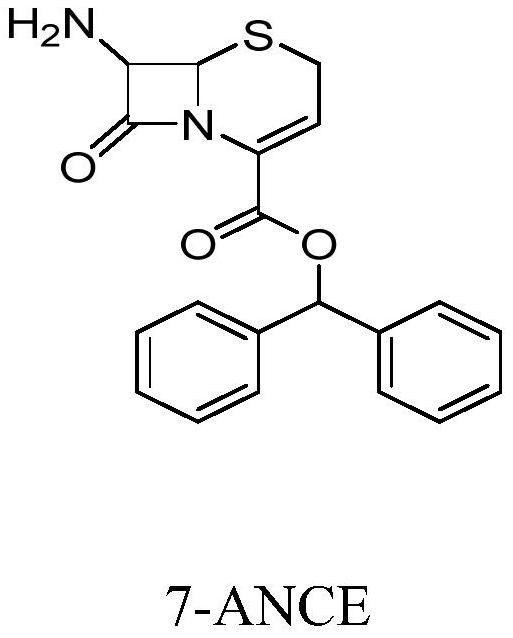
[0046] Step S3: Put the ammonium salt into 150ml of water, use 10% sodium bicarbonate solution to adjust the pH value to 7.6, control the temperature at 25°C and keep stirring for 2 hours, filter, and dry under reduced pressure at 50°C to obtain 7-ANCE, a white solid of 36.1 g.
Embodiment 3
[0048] Step S1: Add 50.2g (0.1mol) of compound A and 400mL of dichloromethane into a 1000mL reaction flask, stir for 20min, and cool down to -20°C; add 62.5g (0.30mol) of phosphorus pentachloride, stir for 10 minutes and drop 27.9 g (0.30 mol) of 2-methylpyridine was added, and the temperature was controlled at -15°C to keep stirring and react for 2 hours.
[0049] Step S2: After the reaction, control the temperature below -10°C and add HBr isopropanol solution (300ml, acid mass concentration 13%) dropwise, control the temperature at 0°C, keep stirring for 3 hours, filter after the stirring, and filter out the ammonium salt.
[0050] Step S3: Put the ammonium salt into 200ml of water, adjust the pH value to 8.3 with 10% ammonia solution, control the temperature at 25°C and keep stirring for 2 hours, filter, and dry under reduced pressure at 50°C to obtain 7-ANCE, 36.0g of white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com