Semiconductor photocatalytic water splitting method capable of inhibiting reverse reaction
A semiconductor and photocatalytic technology, applied in chemical instruments and methods, physical/chemical process catalysts, inorganic chemistry, etc., can solve problems such as unfavorable and complete water decomposition efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]Preparation of precious metal / semiconductor photocatalyst:
[0023]1 gram of agriculture-mixed zinc oxide and gallium oxide powder (zinc gallium gas ratio of 2.5) was placed in a tube furnace, raised to 750 ° C for 10 h in 150 ml / min ammonia stream, fell to room temperature, and zone containing 0.2 The% oxygen is blown for 2 hours, and it is taken from GaN-ZnO, and the detailed group is Zn.0.18GA0.82O0.18N0.82. The 100 mg GaN-ZnO powder was placed in 100 ml of deionized water, and the ultrasound was dispersed for 5 min, and 0.4 ml of RH content was 1 mg / ml of sodium chloride solution, vacuum 30min, and 300-w xenon lamp visible light (wave tensor than 420 nm) After 4 h, then washed with abuse, dried at 50 ° C for 12 h, and the RH / GaN-ZnO photocatalyst was obtained.
Embodiment 2
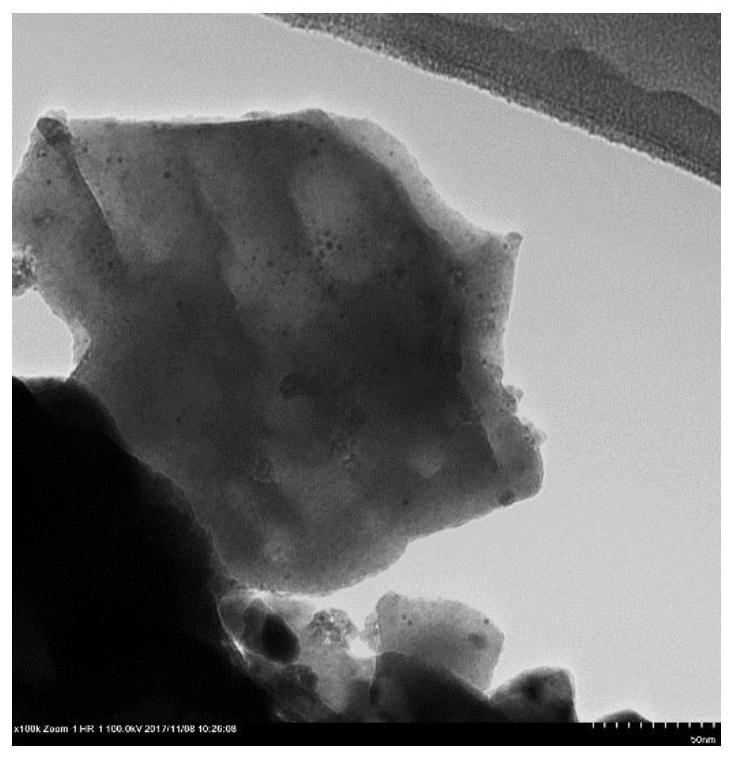
[0025]150 mg of the RH / GaN-ZnO photocatalyst powder of Example 1 was placed on a stainless steel substrate, and it was covered with a stainless steel net to prevent the powder from being blown away, and then put it into the ALD reaction chamber. After the vacuum, the powder first exposed 60s in a trimethyl aluminum atmosphere and then exposed 360S in a nitrogen gas stream, and then exposed 60s in water vapor, and finally exposed 360s in a nitrogen gas stream. Repeat the ALD cycle, the number of ALD cycles is 2, 5, 10, and three photocatalyst powder products are obtained. Finally, the catalyst powder was subjected to 30 min after 400 ° C, respectively, and the photocatalyst XAL was obtained.2O3 / RH / GaN-ZnO (ALD cycle number x = 2, 5, 10, the corresponding surface modified oxide content is 0.016%, 0.04%, 0.08%), respectively. Photocatalyst 5Al2O3 / RH / GAN-ZnO size is shown in Fig. 1 (a), which is visible to the tetramid, and RH nanoparticles are still evenly distributed over GaN-...
Embodiment 3
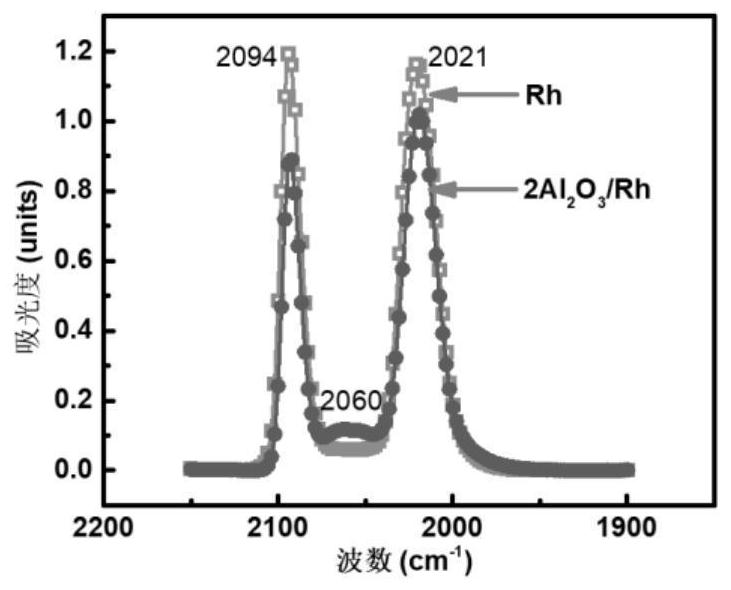
[0027]5Al of RH / GaN-ZnO and Example 2 in Example 12O3 / RH / GaN-ZnO photocatalyst conducts CO suction anime reflection infrared spectrum character, as shown in Fig. 2 (a). After the modification, the CO adsorbed on the low positioning point corresponding to the corner position, indicating Al2O3It is preferentially occupied by the corner of the metal nanoparticles, thereby obtaining the structural characteristics of the photocatalysts of the metallic catalyst in Examples 1 and Example 2. This example only gives Al in RH2O3The structural characteristics of the obtained photocatalyst, but the scope of the invention is not limited thereto. People in the art can easily replace RH to other PT, PD and other precious metals, will Al2O3Extend to SIO2, MGO, TIO2Other oxides. In order to further illustrate the structural characteristics, the corresponding model diagram is shown in the precious metal nanoparticle model and a modified site of the modification in Fig. 2 (b). It should be noted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com