Amide derivative neutral mitochondria fluorescent markers as well as preparation method and application thereof
A technology of amide derivatives and fluorescent markers, applied in the field of fluorescent markers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The general procedure for the synthesis of dyes 2a-e is as follows:
[0066] Take compound 2 (1.0 mmol, 357.2 mg), one of the amide compounds 1a-e (1.0 mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride ( Pd(dppf)Cl 2 , 0.03 mmol, 22 mg) and potassium phosphate (K 3 PO 4 , 2.5 mmoles, 557.5 mg) were dissolved in 15.0 milliliters of 1,4-dioxane (1,4-dioxane) solvent; then the reaction system was replaced by nitrogen three times, then refluxed for 8 to 10 hours, and TLC detected the reaction progress; After cooling down to room temperature, the reacted mixture was suction-filtered, and the filtrate was removed from the solvent by a rotary evaporator, and purified by column chromatography (eluent: dichloromethane / methanol (100 / 1, v / v)) after separation. Dye 2a-e.
[0067] Specifically, the specific synthetic raw materials, yields, and structural characterization results of each product are as follows:
[0068] Dye 2a (262.0 mg) was prepared from compoun...
Embodiment 2
[0074] The general procedure for the synthesis of dyes 3a-e is as follows:
[0075] Take compound 3 (0.5 mmol, 222.1 mg), one of the amide compounds 1a-e (0.6 mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride ( Pd(dppf)Cl 2 , 0.015 mmol, 12 mg) and potassium acetate (AcOK, 1.5 mmol, 334.5 mg) were dissolved in 15.0 ml of 1,4-dioxane (1,4-dioxane) solvent; then the reaction system was replaced by nitrogen three times , heated at 95° C. for 10-12 hours, and TLC detected the reaction progress; after cooling down to room temperature, the reacted mixture was suction-filtered, and the filtrate was removed from the solvent by a rotary evaporator, and column chromatography (eluent: dichloromethane / Methanol (50 / 1, v / v)) gave pure dye after separation.
[0076] Specifically, the specific synthetic raw materials, yields, and structural characterization results of each product are as follows:
[0077] Dye 3a (65.5 mg) was prepared from compound 3 (0.5 mmol, 222.1 mg) a...
Embodiment 3
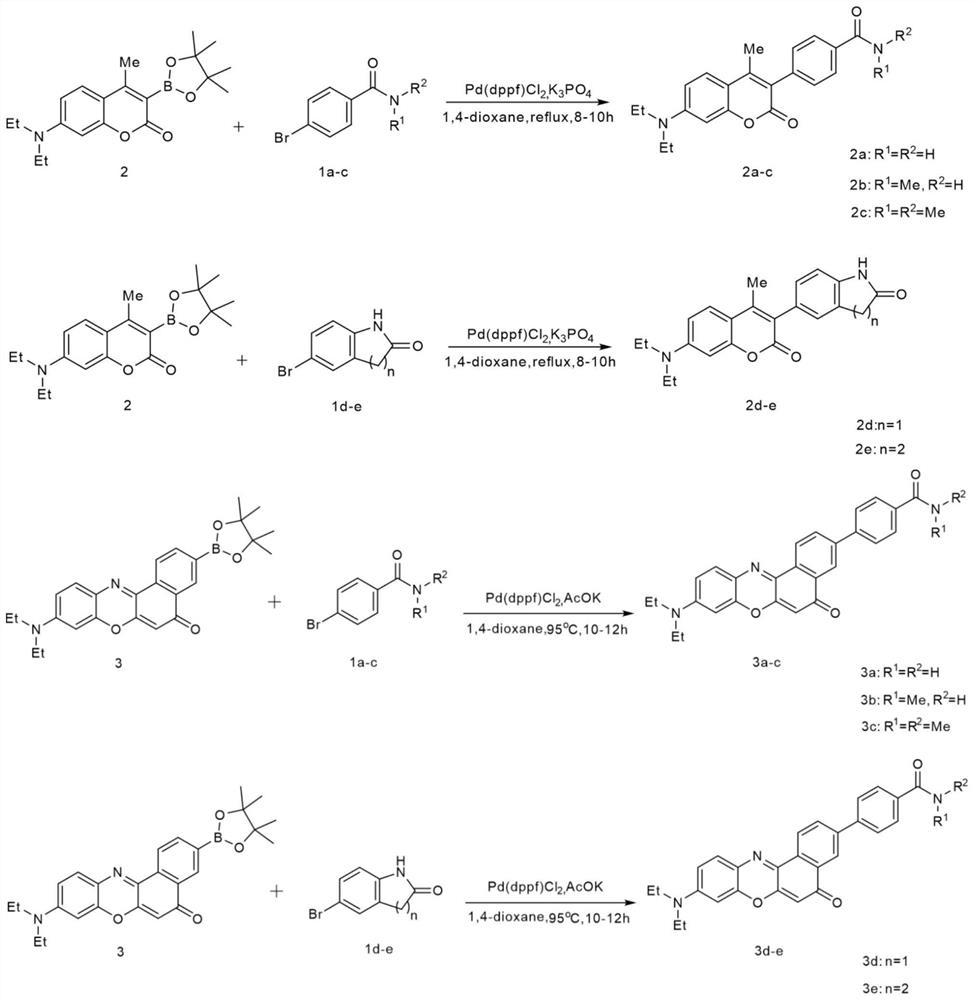
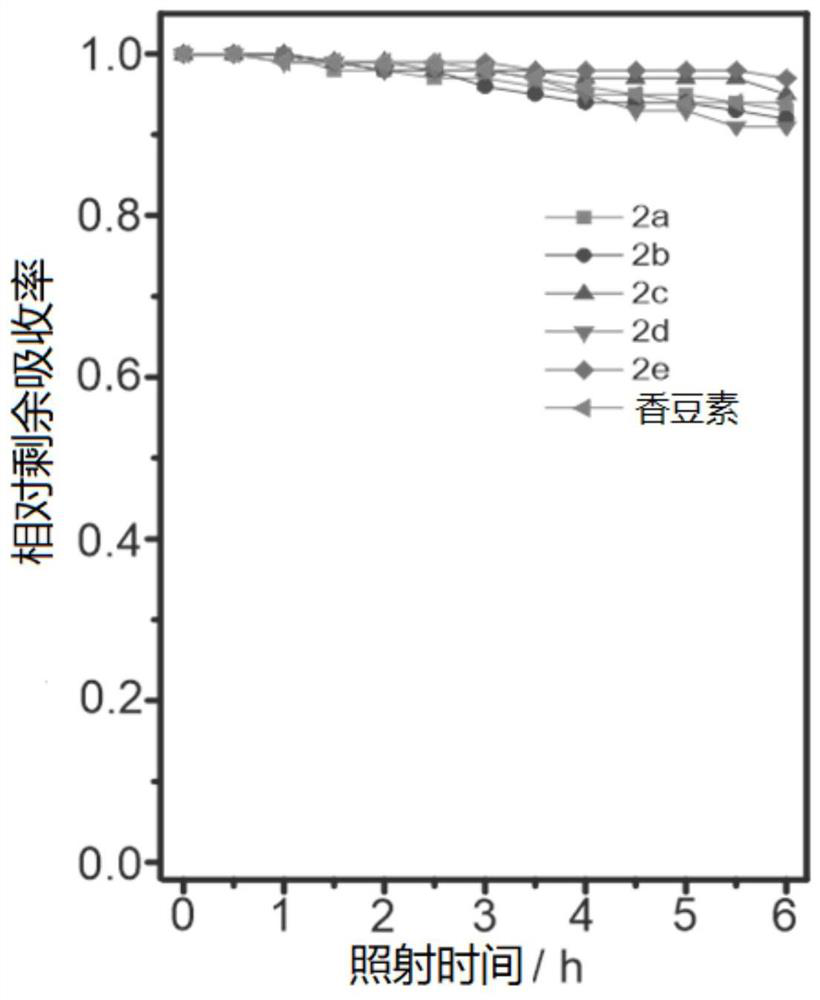
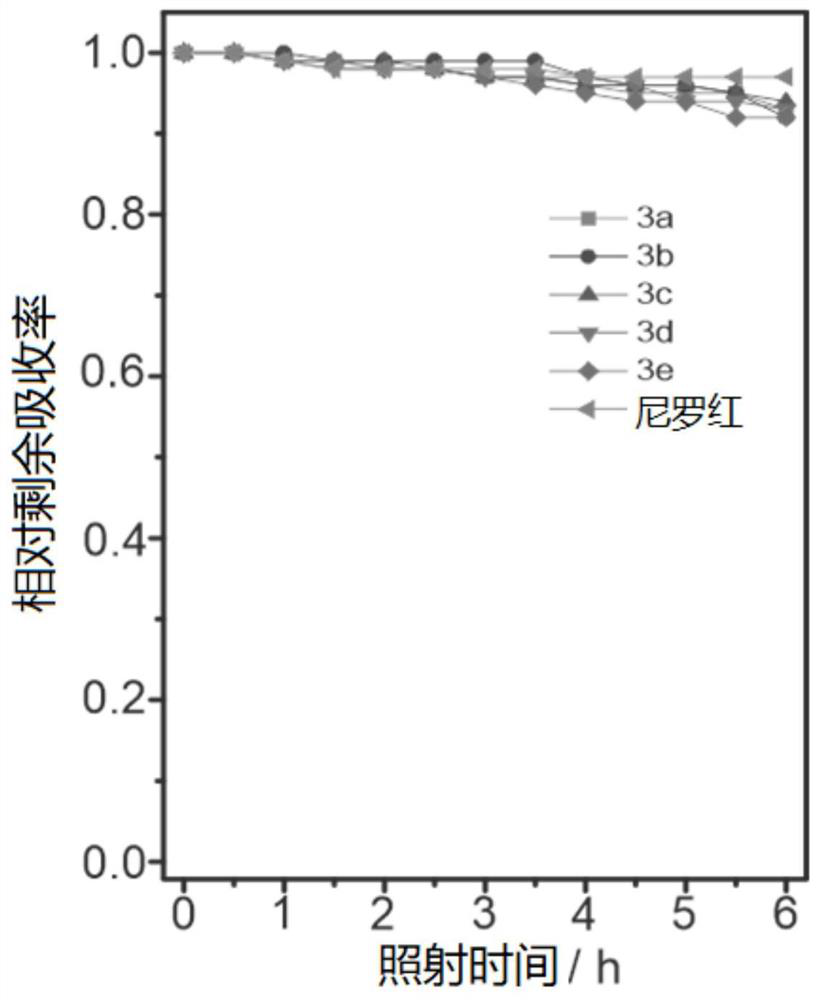
[0083] The above-mentioned prepared dyes 2a-2e, 3a-e (concentration is 10 μM) were tested for photostability, first weighed the corresponding mass of dyes 2a-2e, 3a-e and reference coumarin and Nile red, and weighed them They were respectively dissolved in acetonitrile (concentration: 10 μM), and all samples were irradiated with a Philips iodine-tungsten lamp (500 W), and the distance between the lamp and the sample was set at 25 cm. Place an 8cm thick NaNO between the lamp and the sample 2 (60g.L -1 ) cold trap to remove heat and short wavelength light. Continuous irradiation for 6 hours, in which a UV fluorescence test is performed every half hour. After six hours, the photostability is calculated according to the change of the absorption intensity at different times before and after irradiation to calculate the remaining absorption rate. Such as figure 2 and image 3 As shown, the residual absorption of coumarin after continuous irradiation for 6 hours is 94%, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com